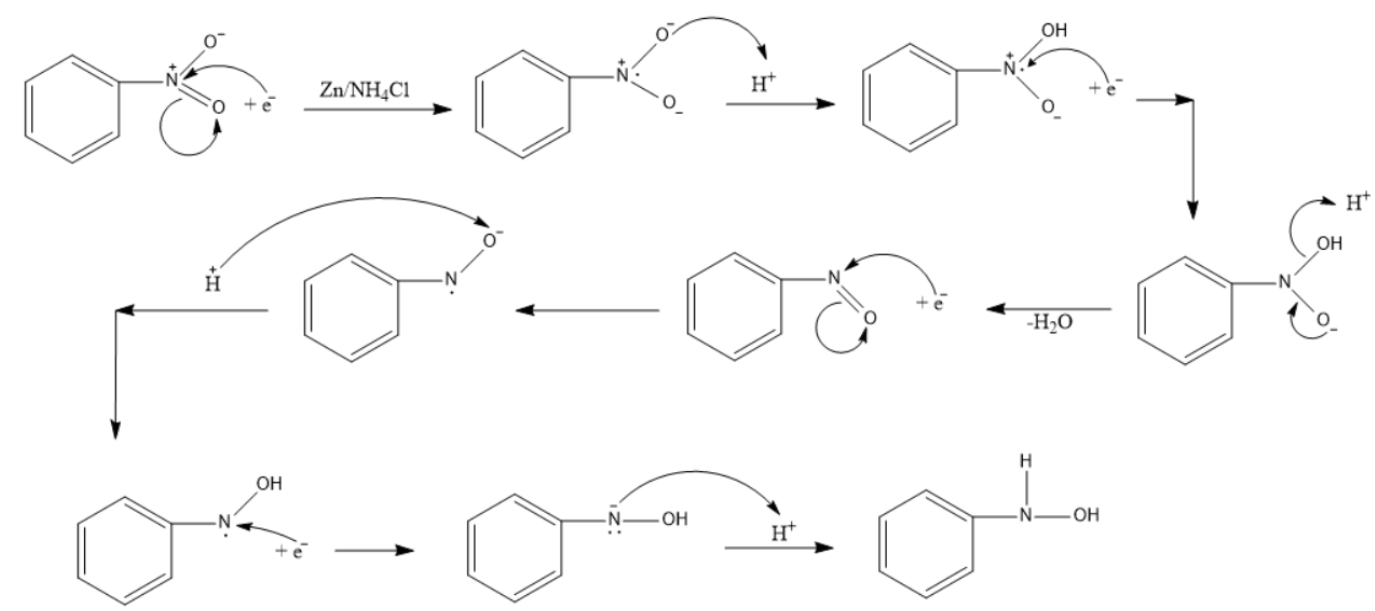
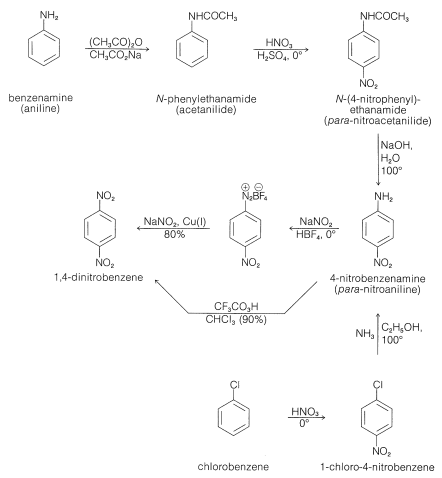
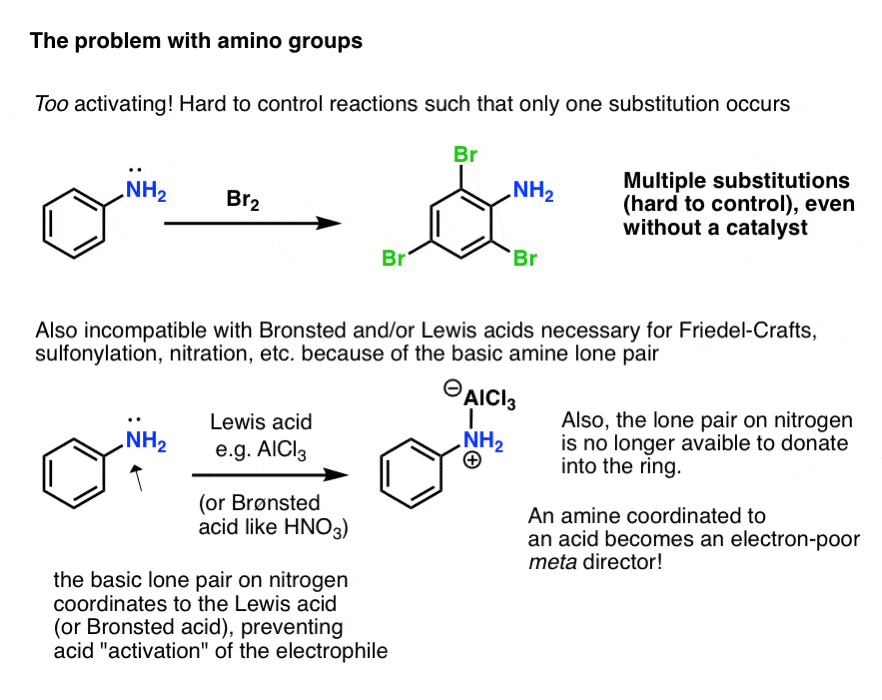
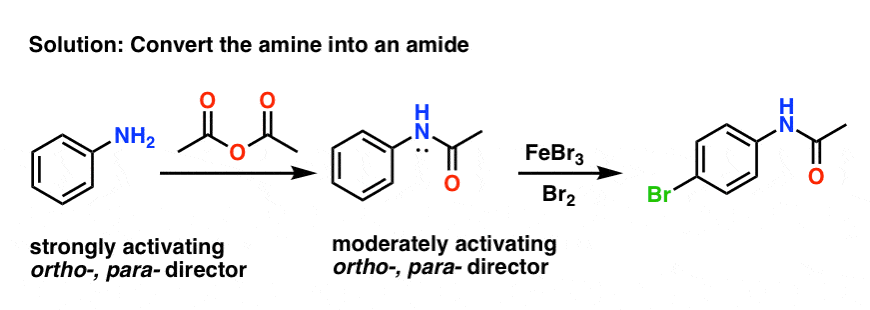
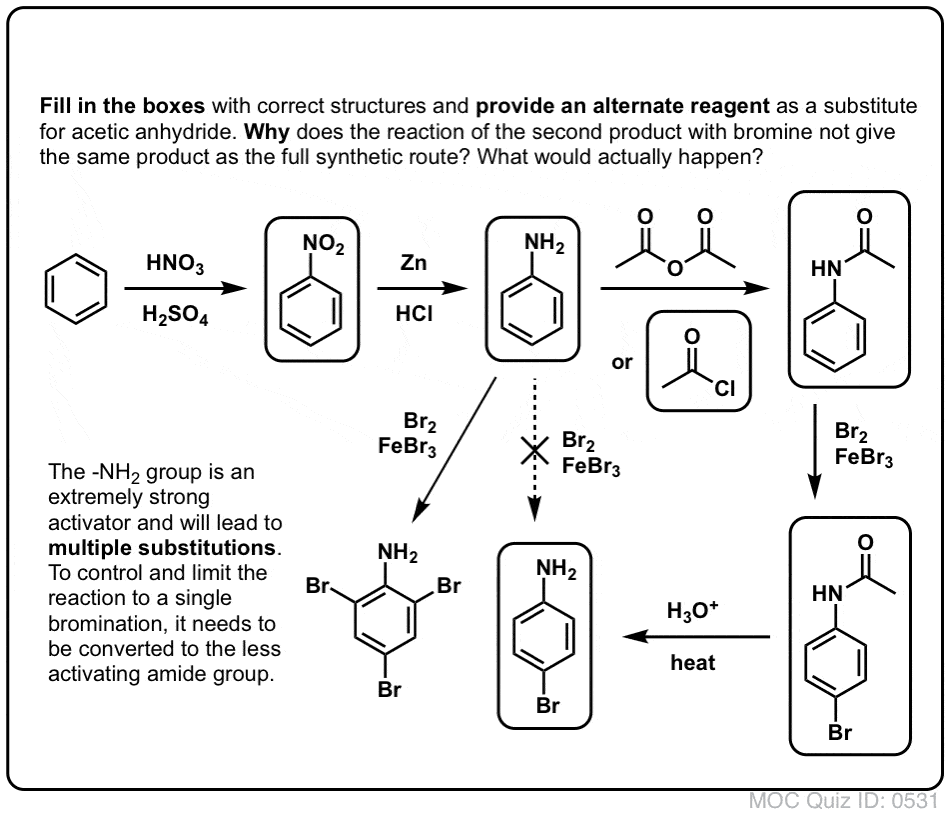
Mechanism for generation of electrons (a) and for generation of OH −... | Download Scientific Diagram

Sciencemadness Discussion Board - Reduction of nitrobenzene by zinc and ammonium chloride - Powered by XMB 1.9.11
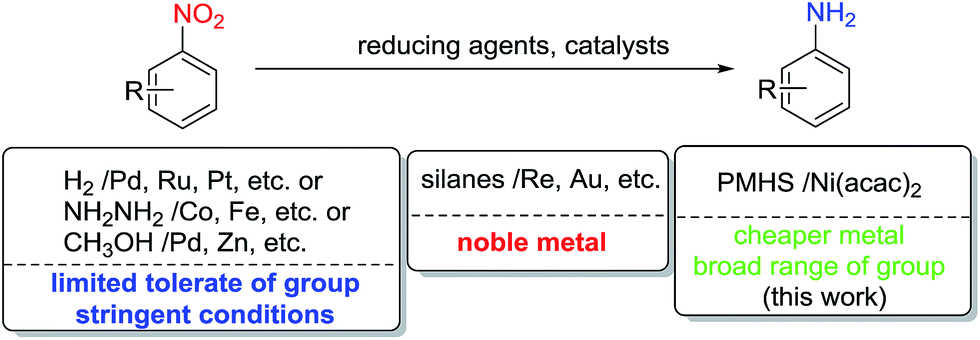
Selective reduction of nitro-compounds to primary amines by nickel-catalyzed hydrosilylative reduction - RSC Advances (RSC Publishing) DOI:10.1039/C5RA17731F

Reduction of aromatic nitro compounds to amines using zinc and aqueous chelating ethers: Mild and efficient method for zinc activation | Semantic Scholar

PDF) Reduction of aromatic nitro compounds to amines using zinc and aqueous chelating ethers: Mild and efficient method for zinc activation

Chemoselective nitro reduction and hydroamination using a single iron catalyst. - Abstract - Europe PMC
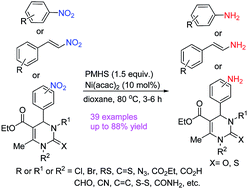
Selective reduction of nitro-compounds to primary amines by nickel-catalyzed hydrosilylative reduction - RSC Advances (RSC Publishing)

Type I nitroreductases reduce the nitro group to generate hydroxylamine... | Download Scientific Diagram

Selective reduction of nitro-compounds to primary amines by tetrapyridinoporphyrazinato zinc (II) supported on DFNS - ScienceDirect

organic chemistry - Nitrobenzene reduction with (tin) Sn catalyst: Why is C-H bond cleavage preferred over O-H bond cleavage? - Chemistry Stack Exchange