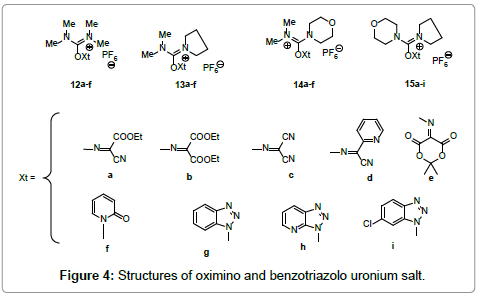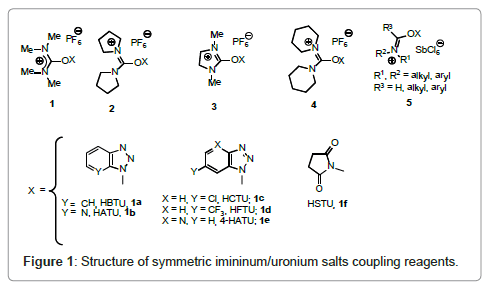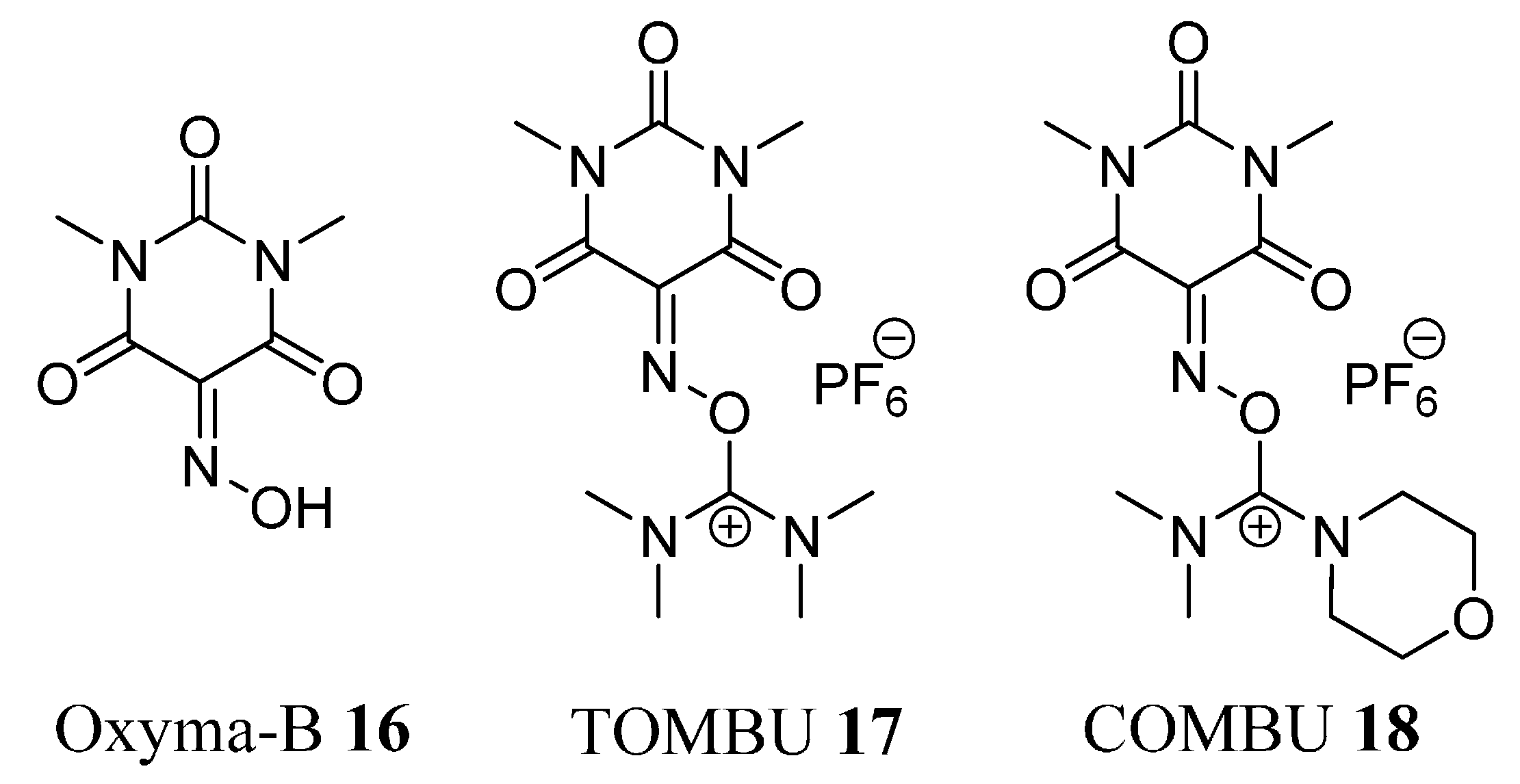
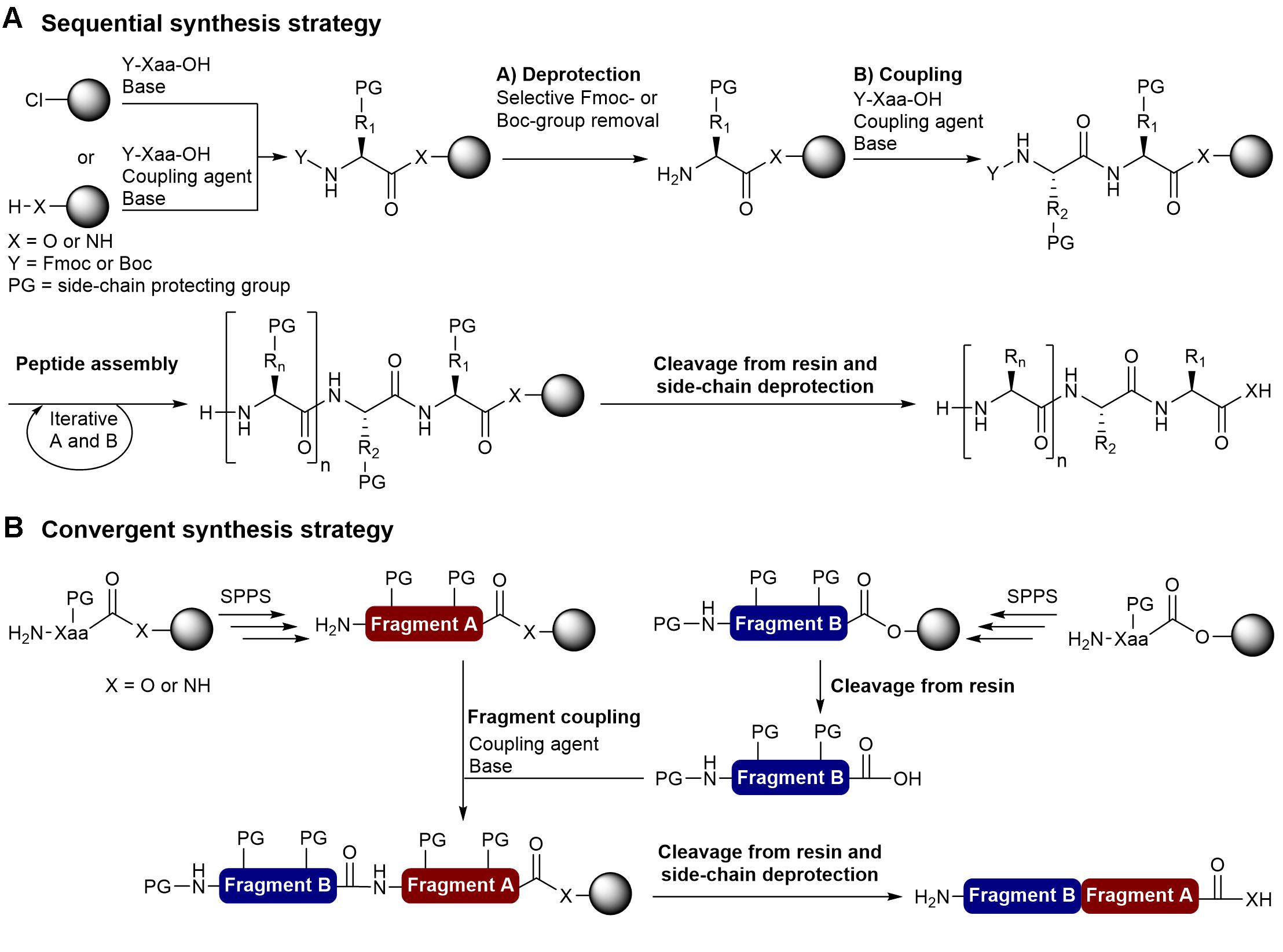
Molecules | Free Full-Text | TOMBU and COMBU as Novel Uronium-Type Peptide Coupling Reagents Derived from Oxyma-B | HTML

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications
![Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole‐Based HOBt and HOAt with a Lower Risk of Explosion[1] - Subirós‐Funosas - 2009 - Chemistry – A European Journal - Wiley Online Library Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole‐Based HOBt and HOAt with a Lower Risk of Explosion[1] - Subirós‐Funosas - 2009 - Chemistry – A European Journal - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/f25812e6-46f3-48f3-8b7c-f247189f2dfd/msch002.jpg)
Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole‐Based HOBt and HOAt with a Lower Risk of Explosion[1] - Subirós‐Funosas - 2009 - Chemistry – A European Journal - Wiley Online Library

Ethyl 2‐(tert‐Butoxycarbonyloxyimino)‐2‐cyanoacetate (Boc‐Oxyma) as Coupling Reagent for Racemization‐Free Esterification, Thioesterification, Amidation and Peptide Synthesis - Thalluri - 2013 - Advanced Synthesis & Catalysis - Wiley Online Library

EP3037430A1 - Improved coupling method for peptide synthesis at elevated temperatures - Google Patents
Frontiers | Recent Progress in the Chemical Synthesis of Class II and S-Glycosylated Bacteriocins | Microbiology
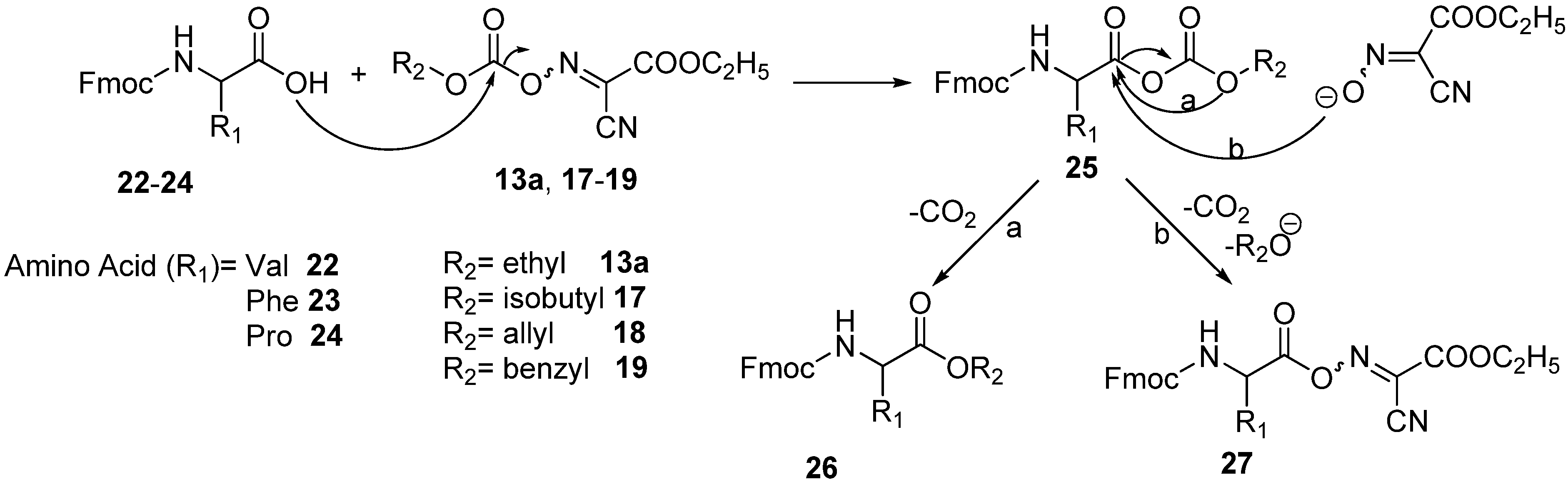
Molecules | Free Full-Text | Oxime-Based Carbonates as Useful Reagents for Both N-Protection and Peptide Coupling | HTML
Use of Oxyma as pH modulatory agent to be used in the prevention of basedriven side reactions and its effect on 2chlorotrityl ch

Oxyma (6)-Based Family of Peptide Coupling Reagents. (Ref. 16,17,19-22) | Download Scientific Diagram