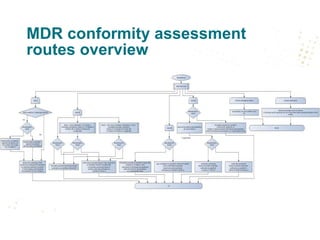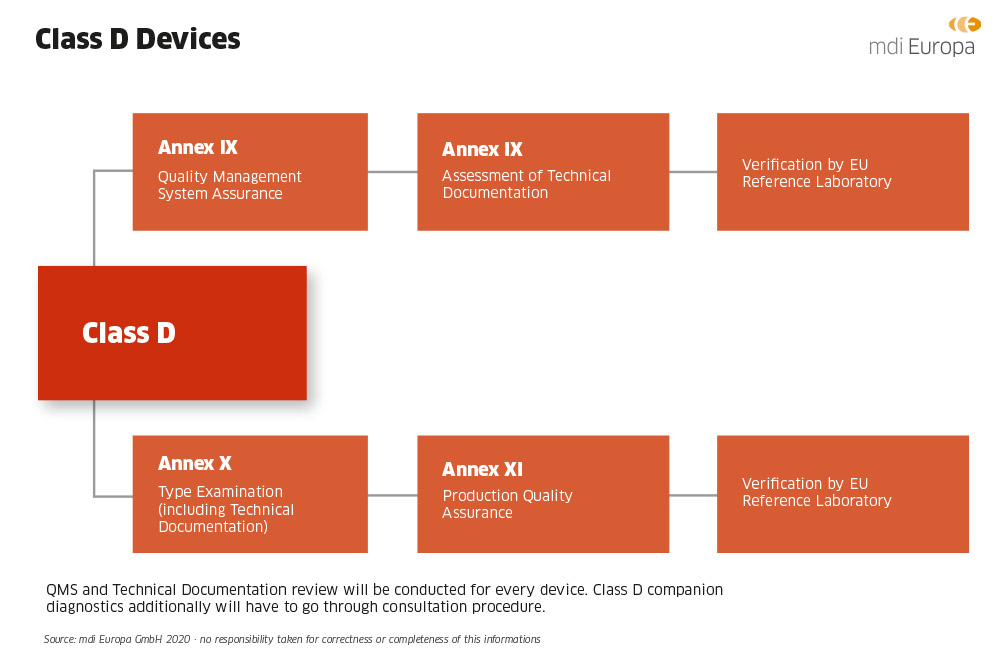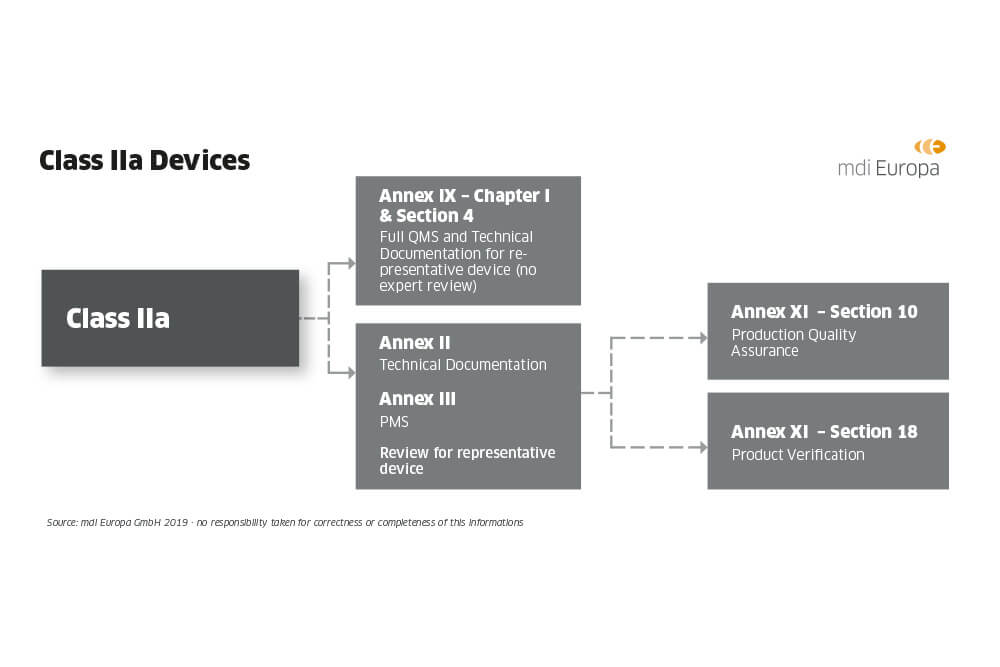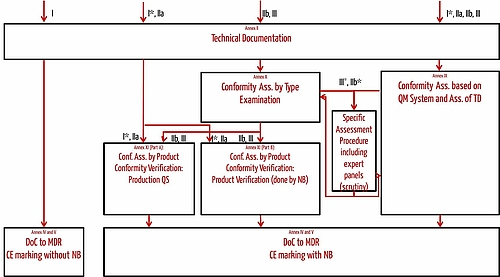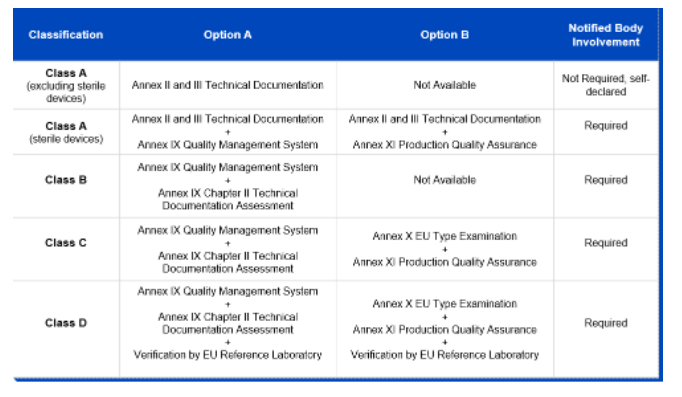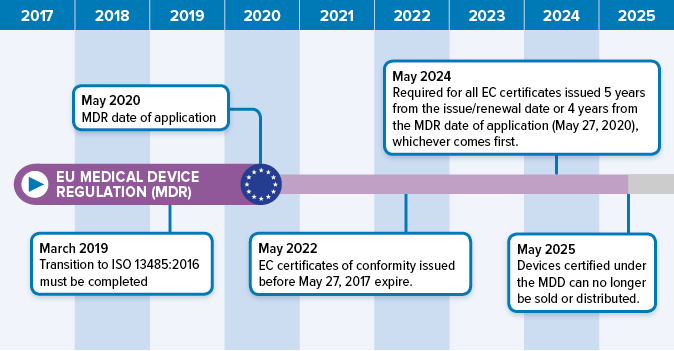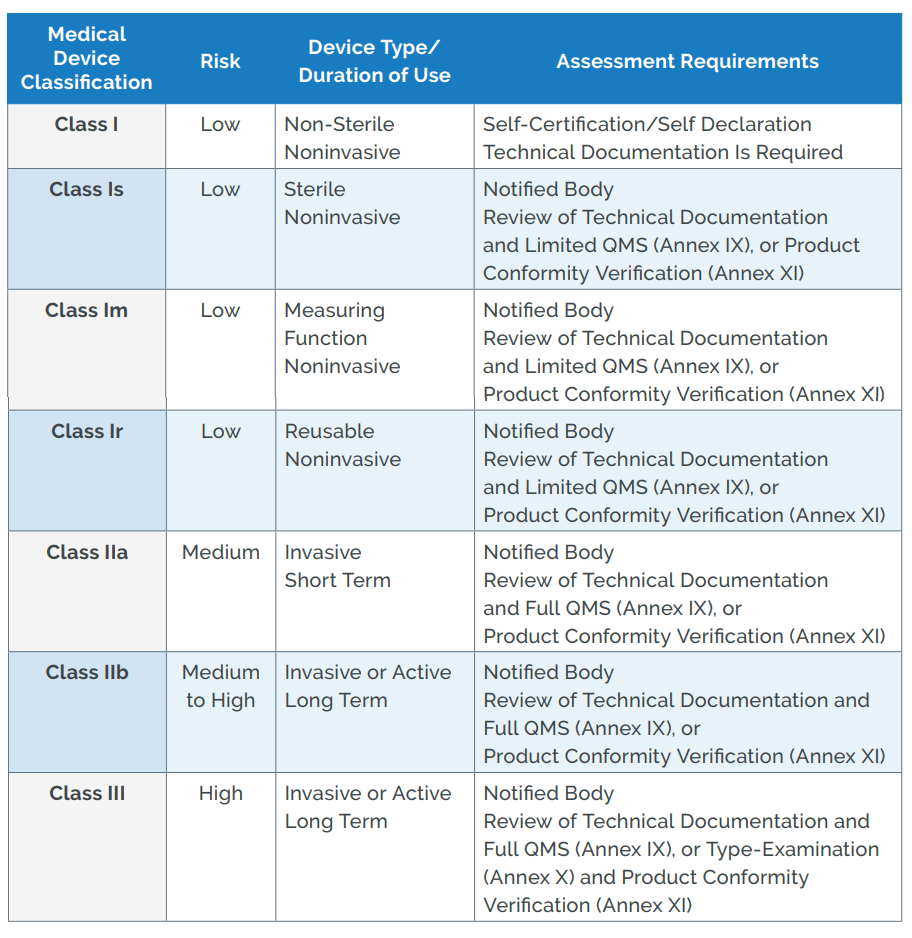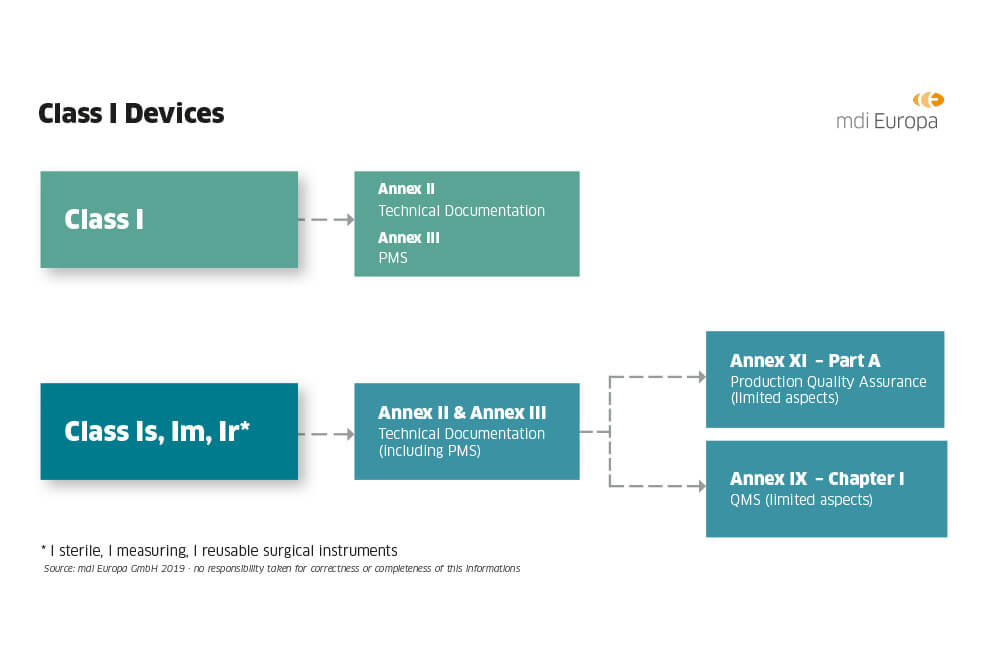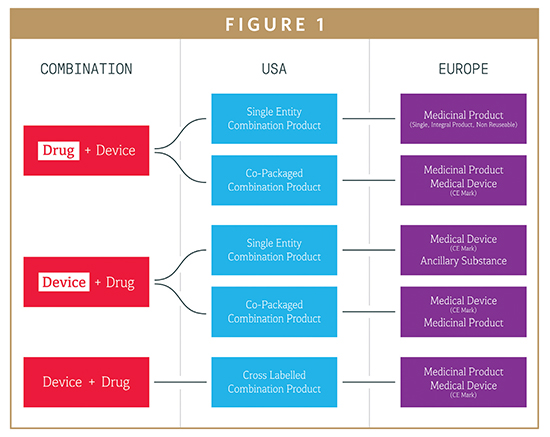
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products

Comparison of the regulatory requirements for custom-made medical devices using 3D printing in Europe, the United States, and Australia