Recombinant replacement therapy for hereditary angioedema due to C1 inhibitor deficiency | Immunotherapy

Inhibition of plasma kallikrein by a highly specific active site blocking antibody. - Abstract - Europe PMC

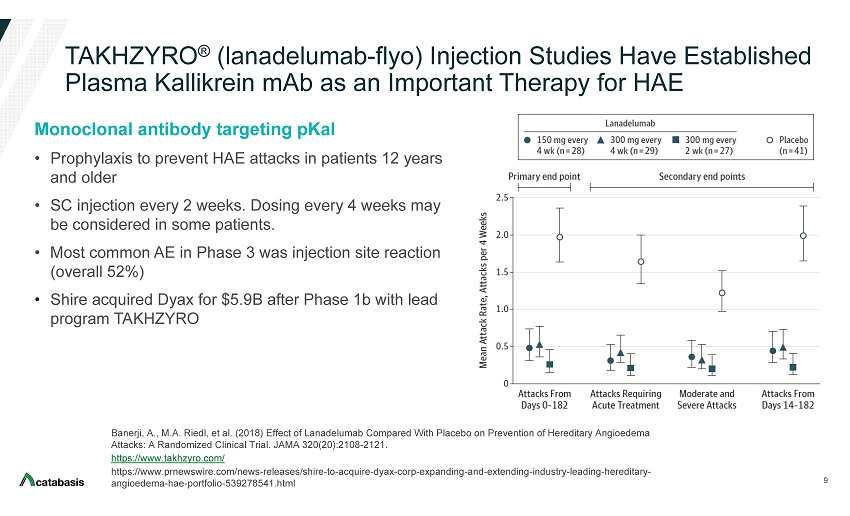
An open‐label study to evaluate the long‐term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension - Riedl - 2017 - Clinical and
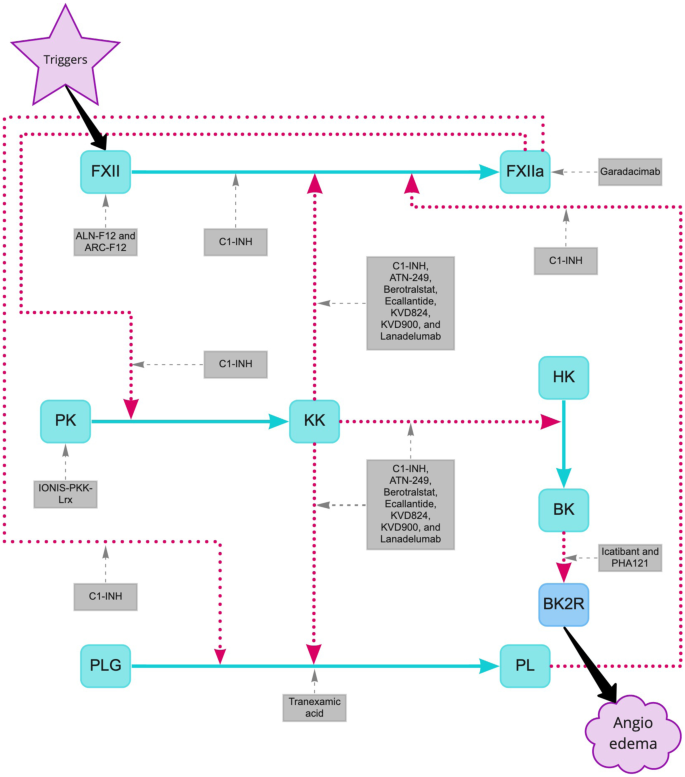
Current and Prospective Targets of Pharmacologic Treatment of Hereditary Angioedema Types 1 and 2 | SpringerLink

Mechanisms of action for therapeutic agents in treating or pre- venting... | Download Scientific Diagram

These highlights do not include all the information needed to use TAKHZYRO® safely and effectively. See full prescribing information for TAKHZYRO®. TAKHZYRO® (lanadelumab-flyo) injection, for subcutaneous useInitial U.S. Approval: 2018

Lanadelumab for the Prophylactic Treatment of Hereditary Angioedema with C1 Inhibitor Deficiency: A Review of Preclinical and Phase I Studies. - Abstract - Europe PMC

Interim Phase 4 Data Support TAKHZYRO® (lanadelumab) as an Effective Treatment to Reduce Attacks in Hereditary Angioedema Patients | Business Wire

Reviewing clinical considerations and guideline recommendations of C1 inhibitor prophylaxis for hereditary angioedema - Anderson - 2022 - Clinical and Translational Allergy - Wiley Online Library

US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema - The Journal of Allergy and Clinical Immunology: In Practice