A one-pot oxidative decarboxylation–Friedel-Crafts reaction of acyclic α-amino acid derivatives activated by the combination of iodobenzene diacetate ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B815227F
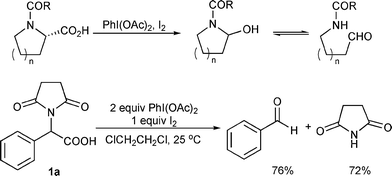
A one-pot oxidative decarboxylation–Friedel-Crafts reaction of acyclic α-amino acid derivatives activated by the combination of iodobenzene diacetate ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B815227F

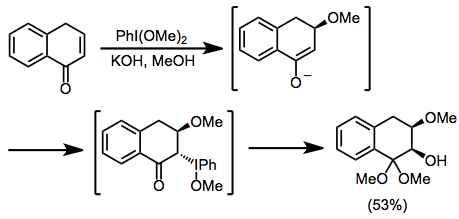
organic chemistry - Mechanism of oxidative dearomatisation with hypervalent iodine - Chemistry Stack Exchange

Synthesis of 3,5-diarylisoxazoles under solvent-free conditions using iodobenzene diacetate - ScienceDirect

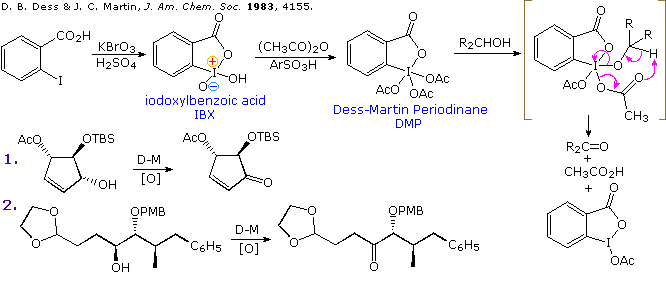
Oxidation of Carbonyl Compounds with Organohypervalent Iodine Reagents - Moriarty - - Major Reference Works - Wiley Online Library

Synthesis of α-sulfonyloxyketones via iodobenzene diacetate (PIDA)-mediated oxysulfonyloxylation of alkynes with sulfonic acids - RSC Advances (RSC Publishing) DOI:10.1039/C7RA11875A

organic chemistry - Mechanism of oxidative dearomatisation with hypervalent iodine - Chemistry Stack Exchange

Novel 2,2,6,6‐Tetramethylpiperidine 1‐Oxyl–Iodobenzene Hybrid Catalyst for Oxidation of Primary Alcohols to Carboxylic Acids - Yakura - 2011 - Advanced Synthesis & Catalysis - Wiley Online Library

Iodine(III) promotes cross-dehydrogenative coupling of N-hydroxyphthalimide and unactivated C(sp3)–H bonds | Communications Chemistry







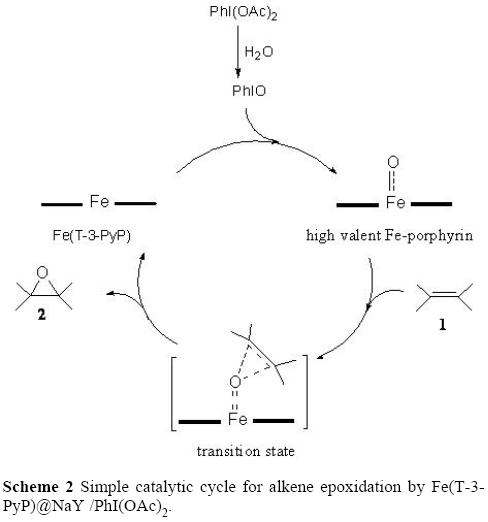
/8F1232C6EE59D103802585F90071D7DB/$file/FI10879_structure.png)