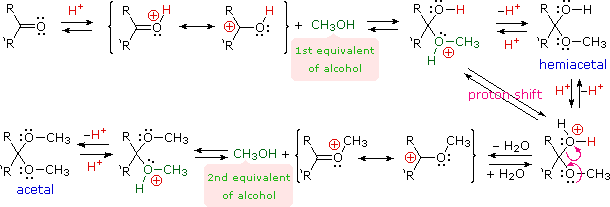
organic chemistry - Why does Brady's reagent include sulfuric acid and methanol? - Chemistry Stack Exchange
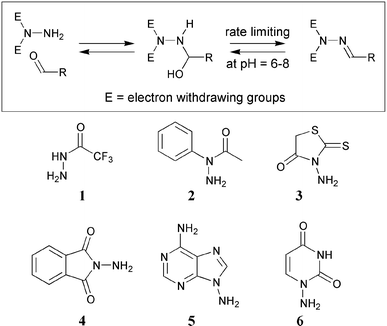
Optimizing the reversibility of hydrazone formation for dynamic combinatorial chemistry - Chemical Communications (RSC Publishing) DOI:10.1039/B211645F

Condensation reaction between hydrazine and carbonyl compounds to form... | Download Scientific Diagram
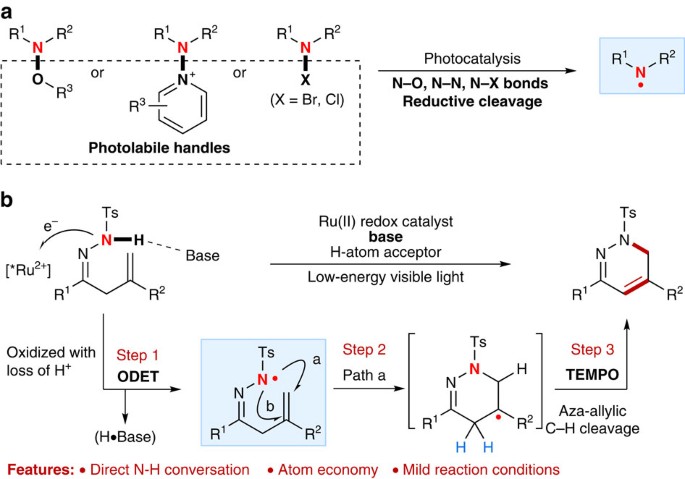
Catalytic N-radical cascade reaction of hydrazones by oxidative deprotonation electron transfer and TEMPO mediation | Nature Communications
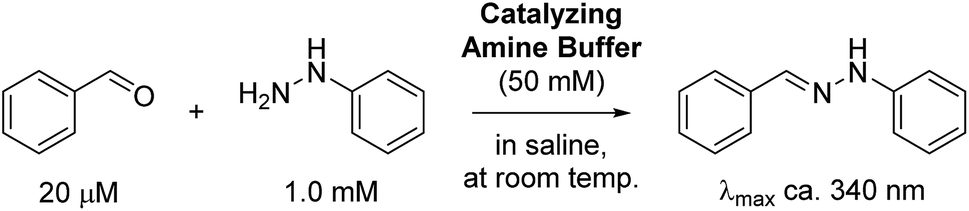
Exceptionally rapid oxime and hydrazone formation promoted by catalytic amine buffers with low toxicity - Chemical Science (RSC Publishing) DOI:10.1039/C8SC01082J

Hydrazone-based switches, metallo-assemblies and sensors - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60385G

![PDF] Hydrolytic stability of hydrazones and oximes. | Semantic Scholar PDF] Hydrolytic stability of hydrazones and oximes. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5dd85edc0941ee0d75a381fc4002f12644b2bc2f/10-Figure5-1.png)
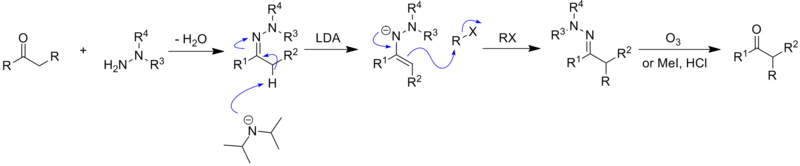
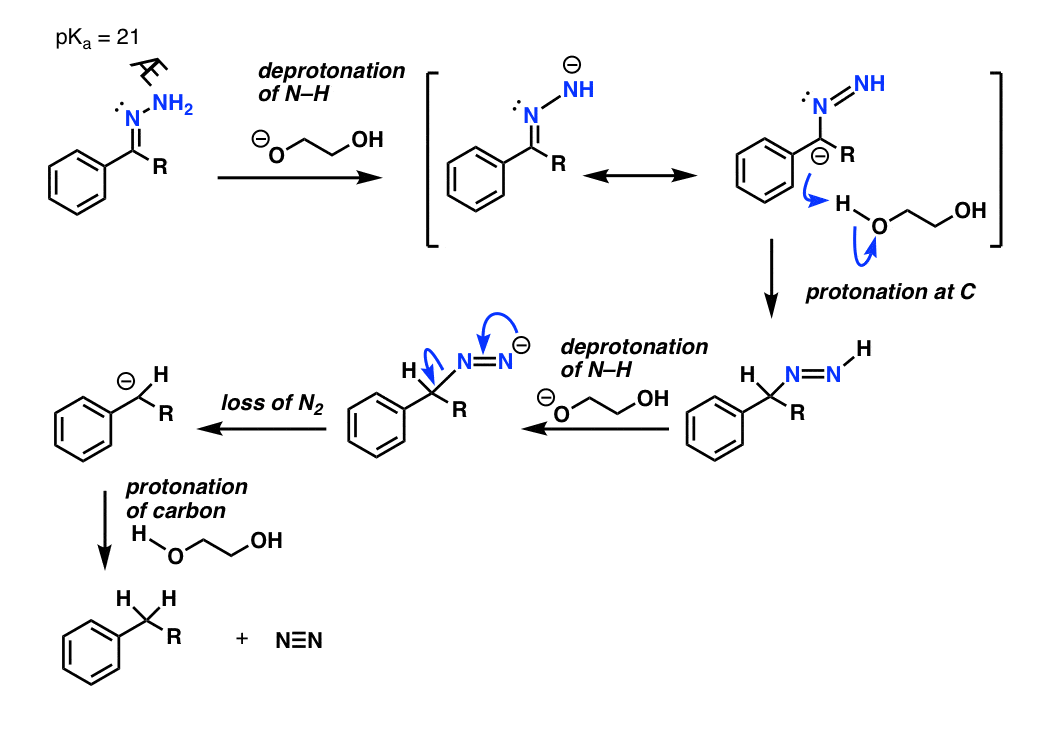

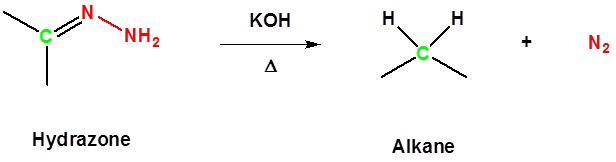
/chapter6/pages33and34/page33and34_files/hydrazoneequation.png)



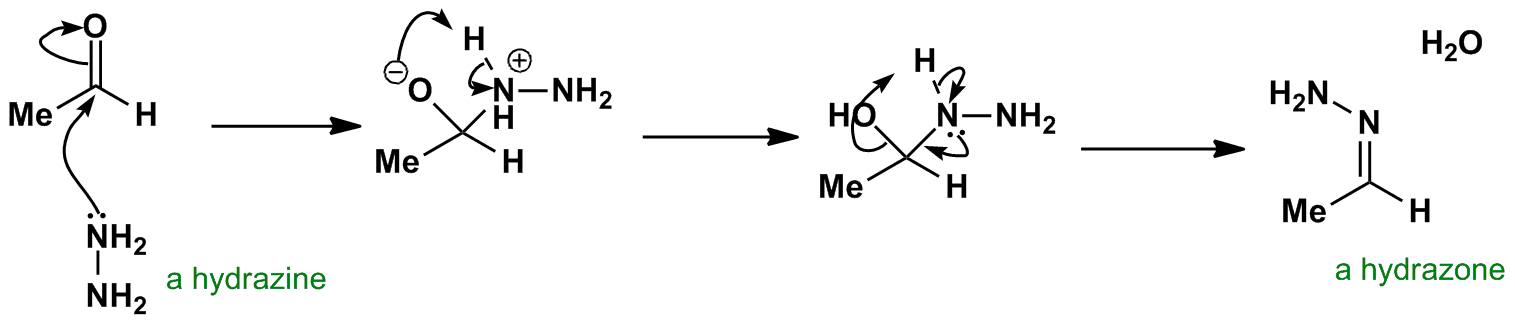

![7 Mechanistic details of hydrazone formation [55]. | Download Scientific Diagram 7 Mechanistic details of hydrazone formation [55]. | Download Scientific Diagram](https://www.researchgate.net/profile/Michael-Breadmore/publication/226521176/figure/fig2/AS:393651999002625@1470865358066/Mechanistic-details-of-hydrazone-formation-55.png)
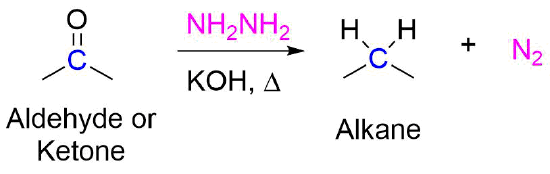

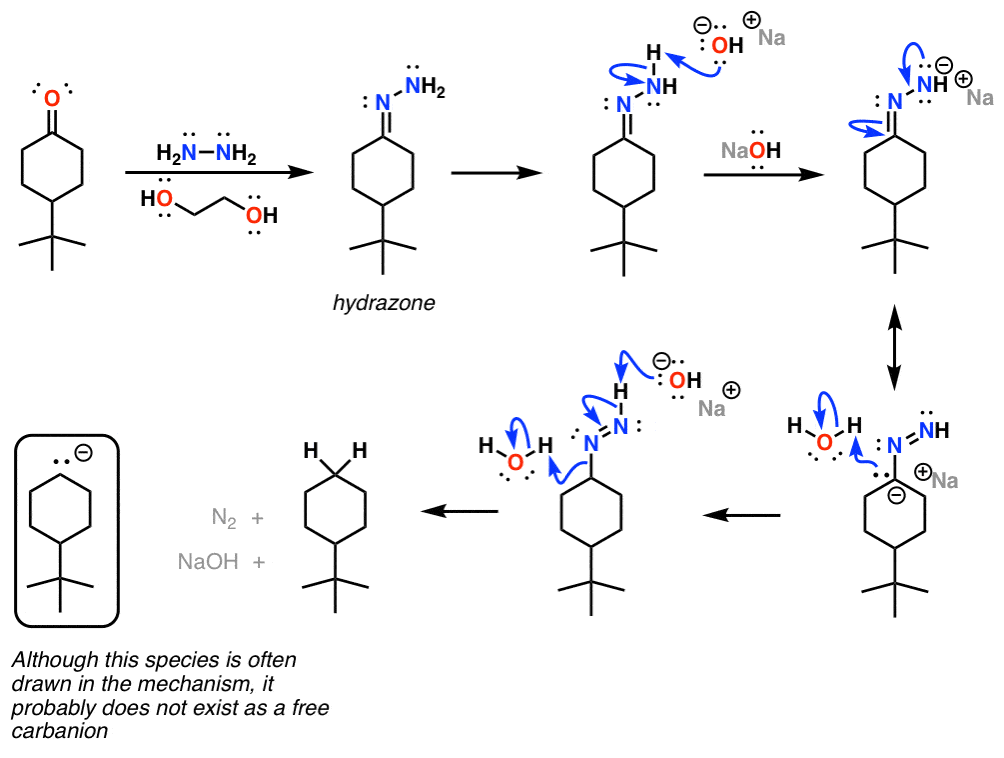
/chapter6/pages33and34/page33and34_files/WolffKishnermechanism.png)