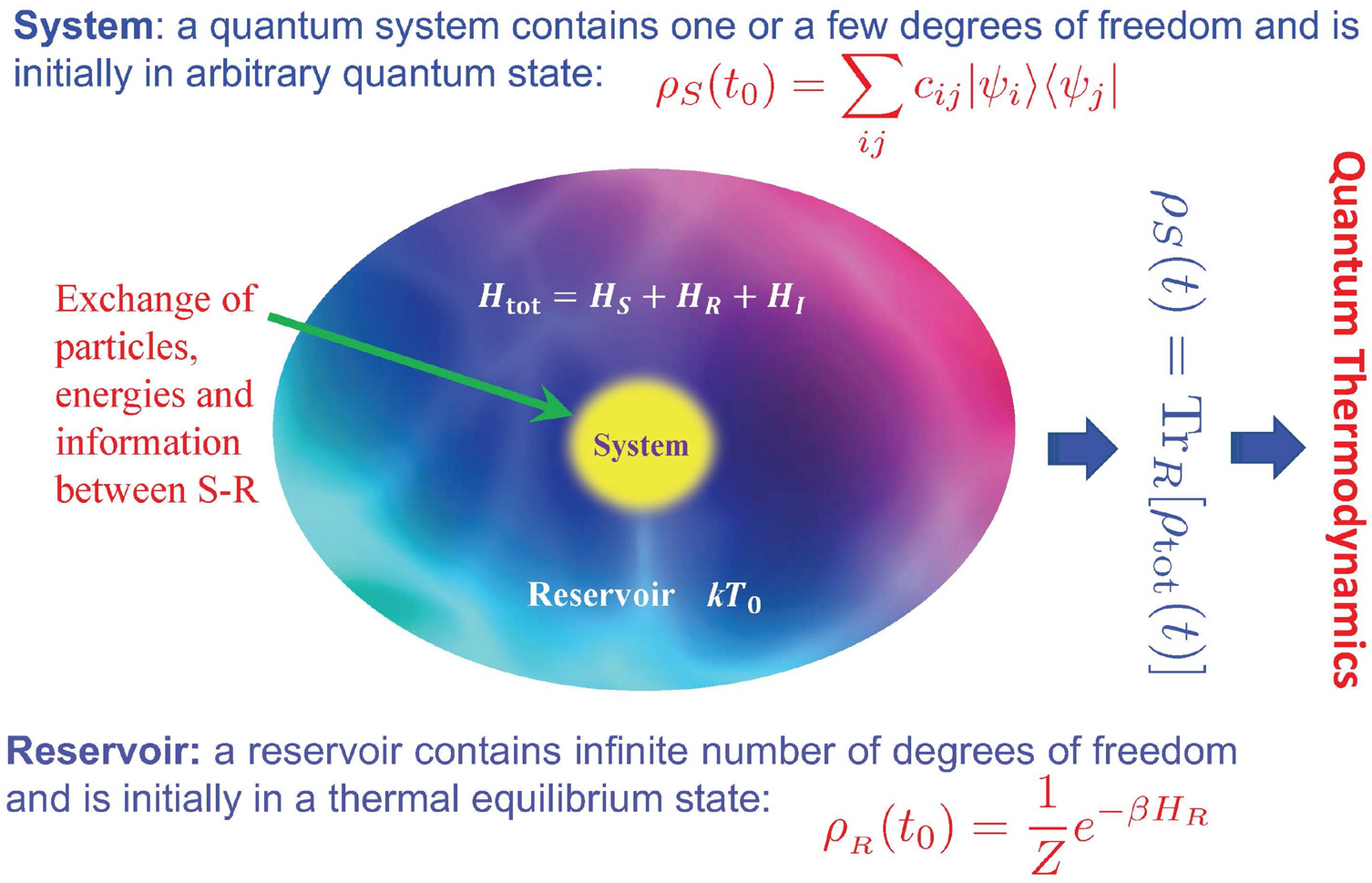
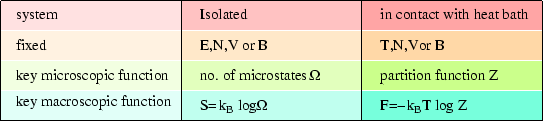
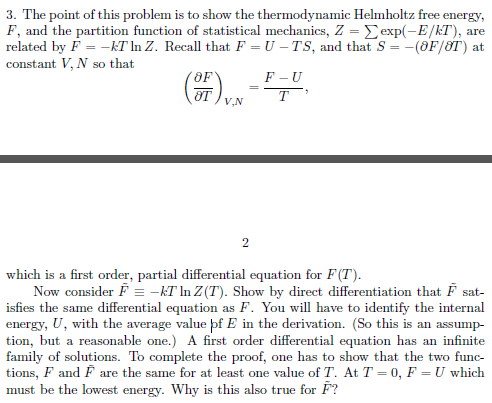
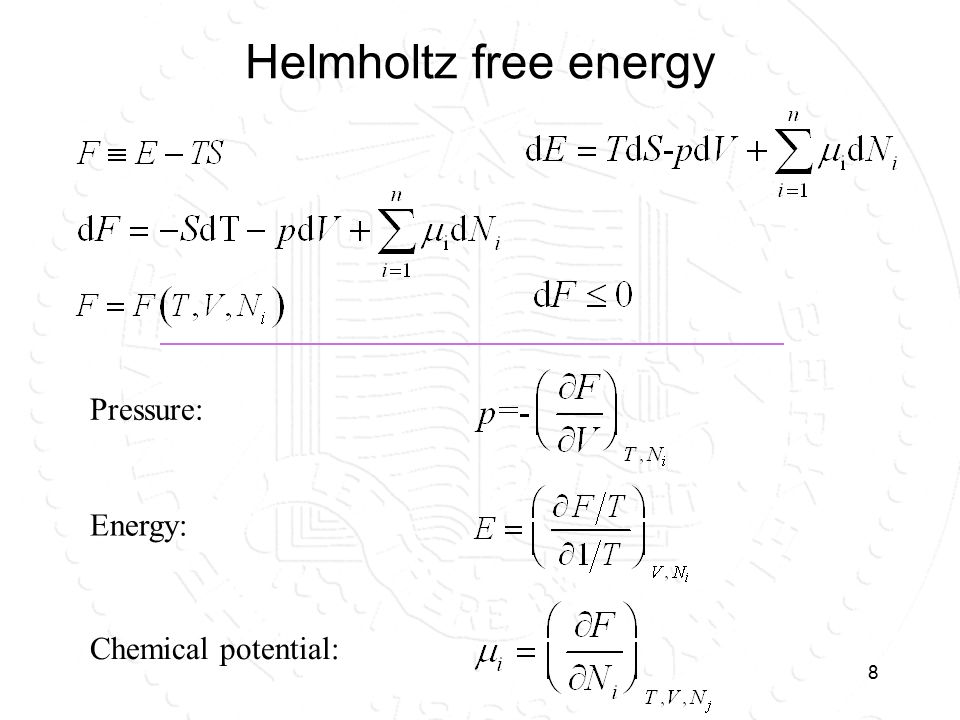
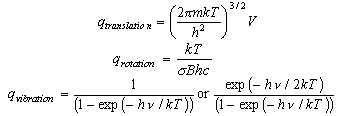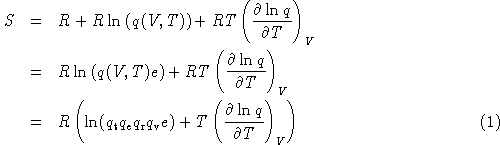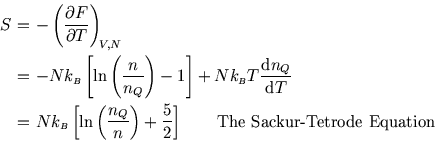homework and exercises - Helmholtz energy in terms of grand partition function - Physics Stack Exchange
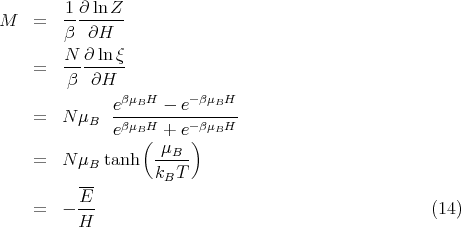
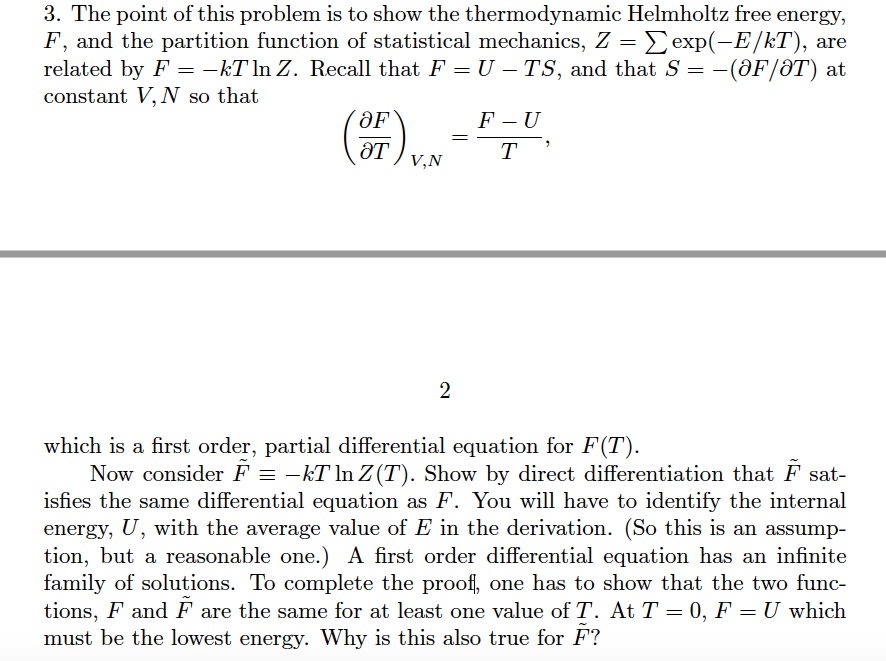
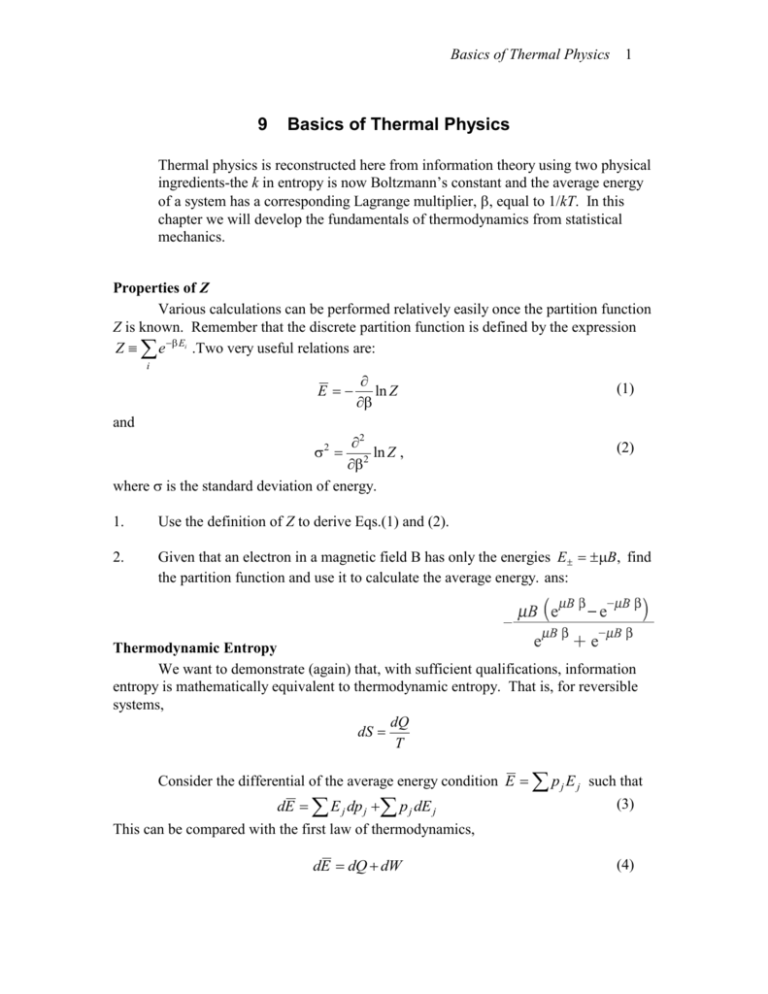
LECTURE 10 Simple Applications of Statistical Mechanics We have seen that if we can calculate the partition function ∑ Z = e-βEr r (1) then we can derive just about anything we want to know from the partition function such as the mean internal energy, the ...

MathType on Twitter: "The #Helmholtz free energy is associated with the work done by a closed system at constant volume and temperature. Given a partition function, this energy is an efficient way