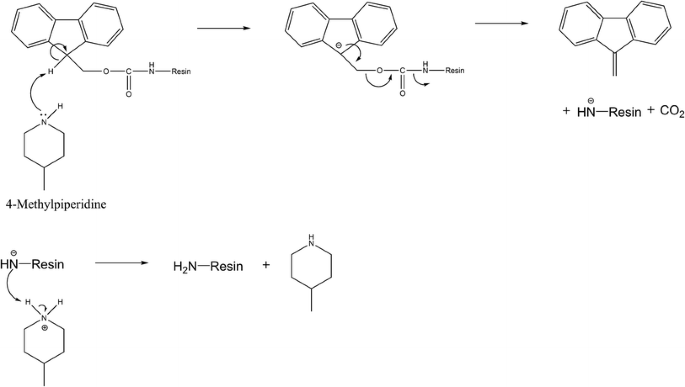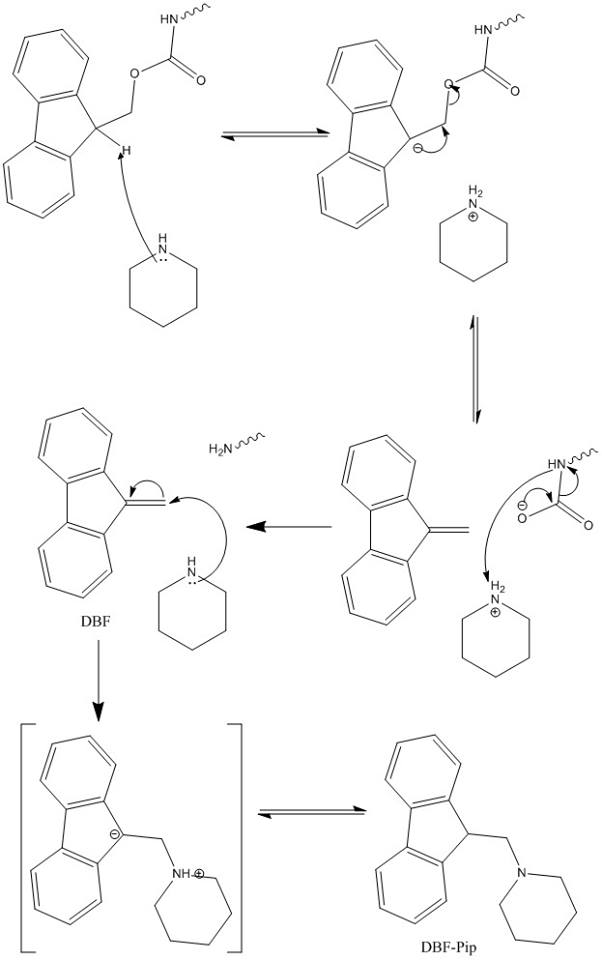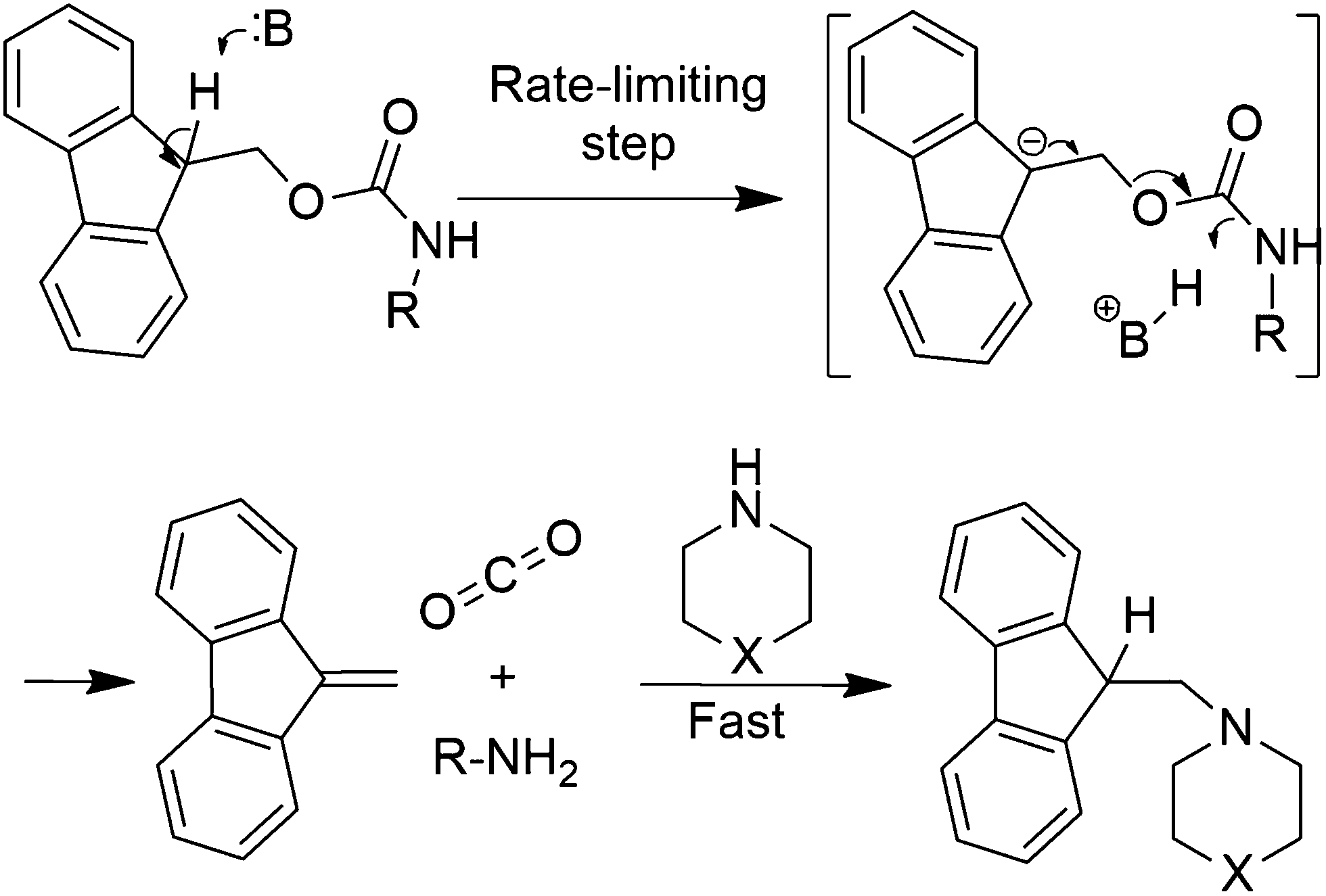
Piperazine and DBU: a safer alternative for rapid and efficient Fmoc deprotection in solid phase peptide synthesis - RSC Advances (RSC Publishing) DOI:10.1039/C5RA23441G
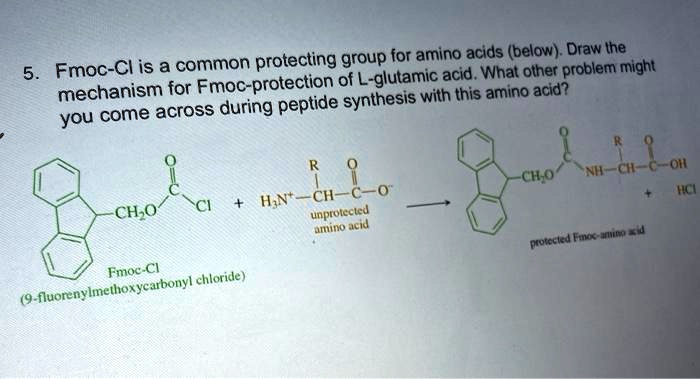
SOLVED:Fmoc-Cl is a common protecting group for amino acids (below) Drblethe acid_ What other problem " 'might mechanism for Fmoc-protection ovntglesanic peptide synthesis = with this amino acid? you come across during
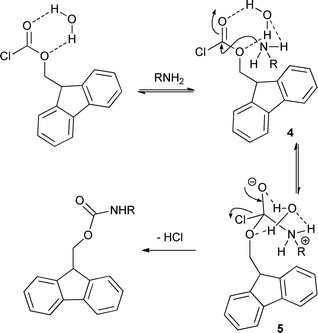
An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media - Green Chemistry (RSC Publishing) DOI:10.1039/C1GC15868F

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications

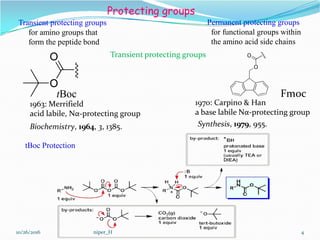
Carpino's protecting groups, beyond the Boc and the Fmoc - El‐Faham - 2020 - Peptide Science - Wiley Online Library

A straightforward method for automated Fmoc-based synthesis of bio-inspired peptide crypto-thioesters - Chemical Science (RSC Publishing) DOI:10.1039/C5SC02630J

Fmoc‐2‐mercaptobenzothiazole, for the introduction of the Fmoc moiety free of side‐reactions - Isidro‐Llobet - 2007 - Peptide Science - Wiley Online Library




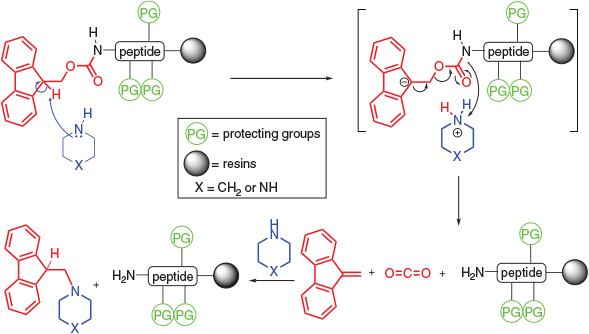

.jpg)