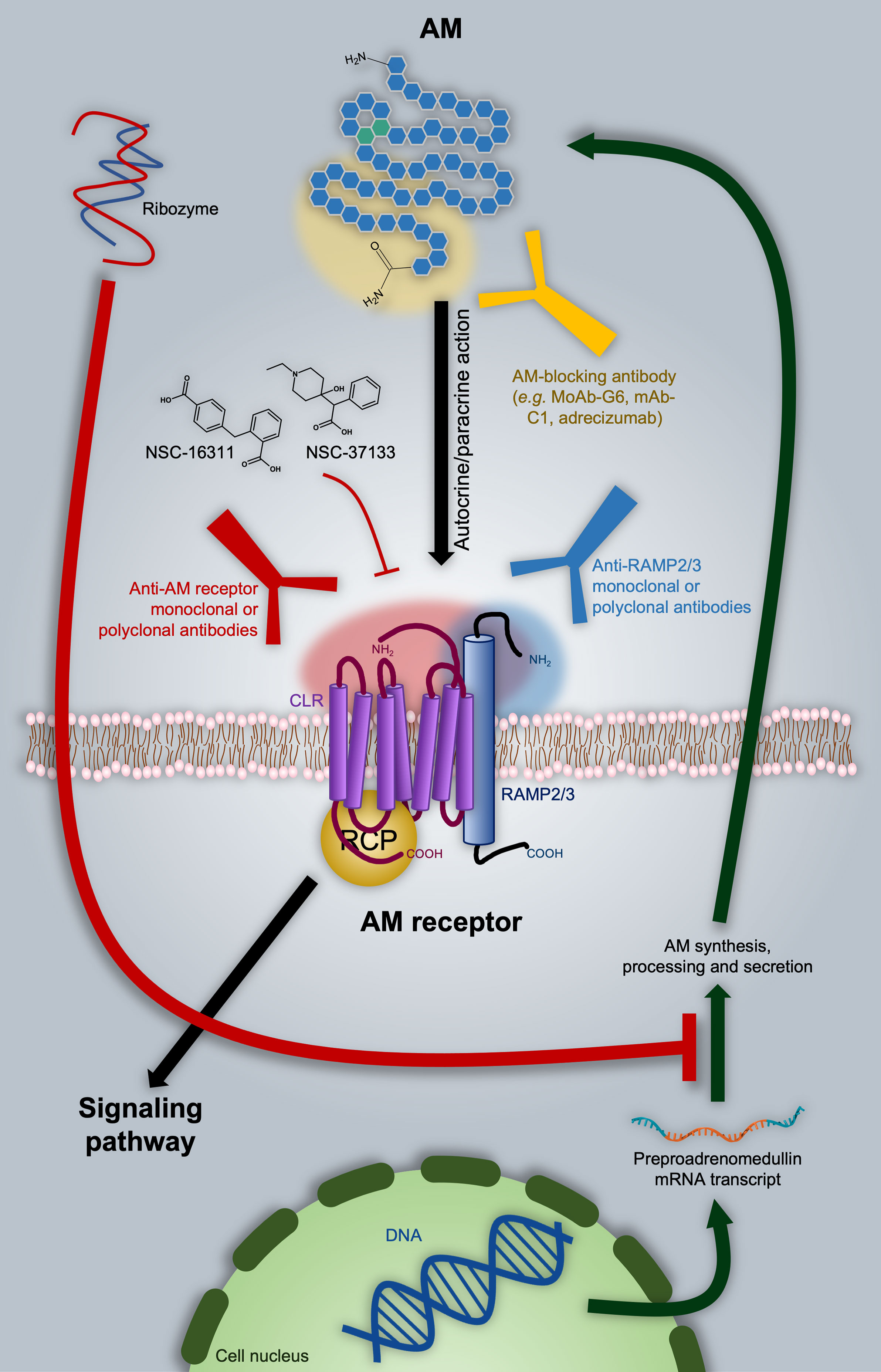
Frontiers | Targeting Adrenomedullin in Oncology: A Feasible Strategy With Potential as Much More Than an Alternative Anti-Angiogenic Therapy | Oncology
1 Cellectar Biosciences NASDAQ: CLRB Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration Statement No. 333 -
Desmoplastic small round cell tumor: a review of main molecular abnormalities and emerging therapy Simple Summary: Desmoplastic

Cellectar Biosciences Inc - EX-99.1 - Corporate Presentation January 2019 NASDAQ: CLRB - January 29, 2019

CLR 131 – A New Approach in Multiple Myeloma Treatment | Multiple Myeloma Treatment| New treatment:CureTalks.com