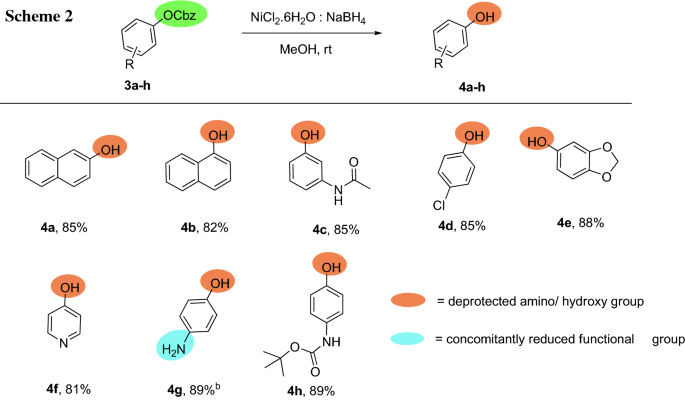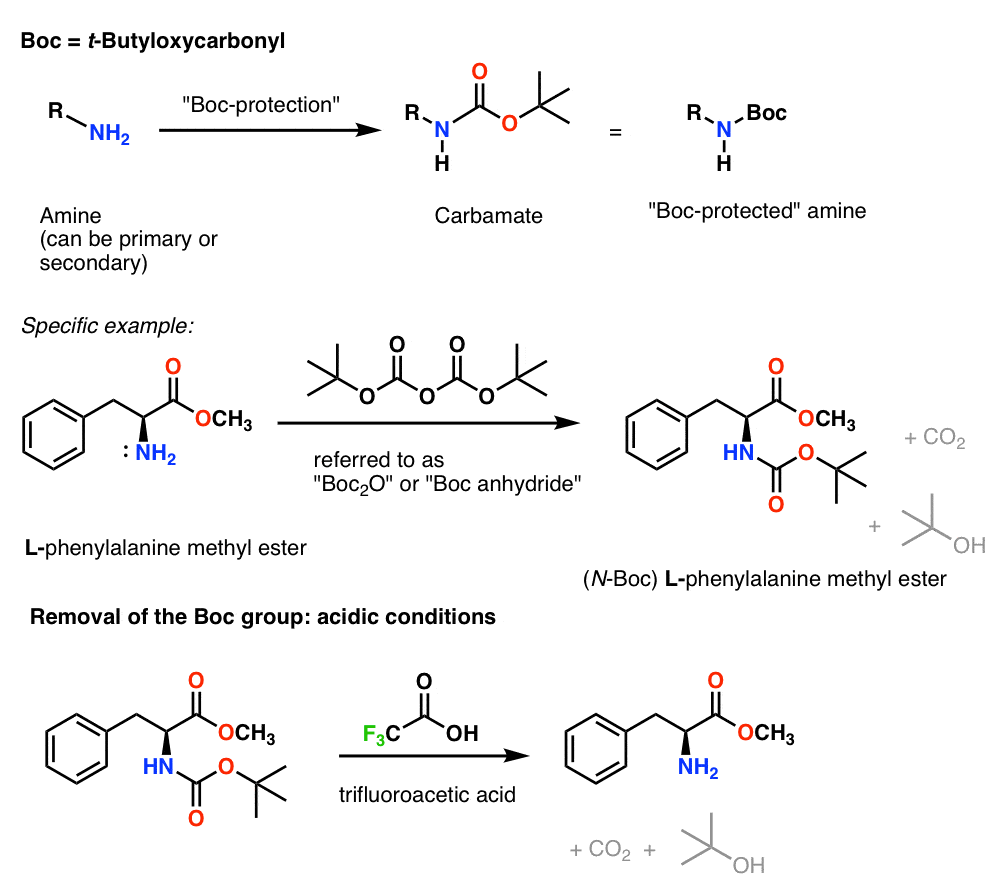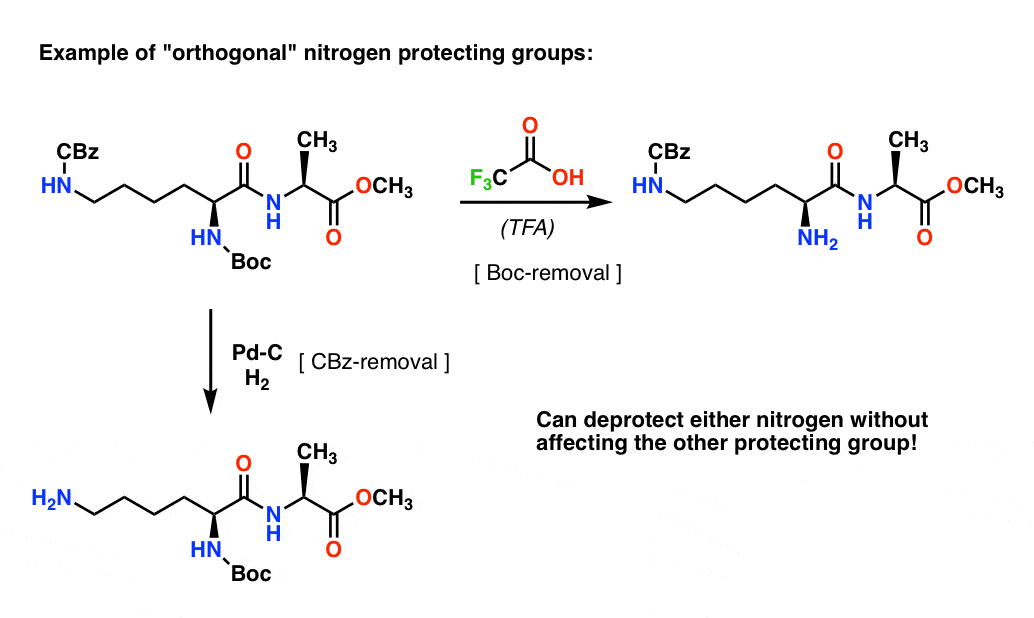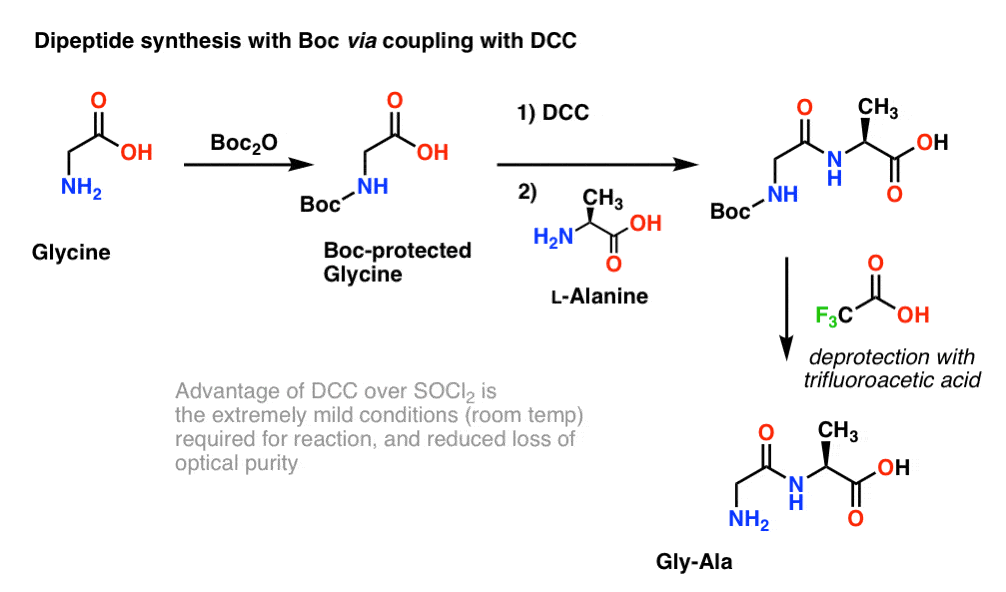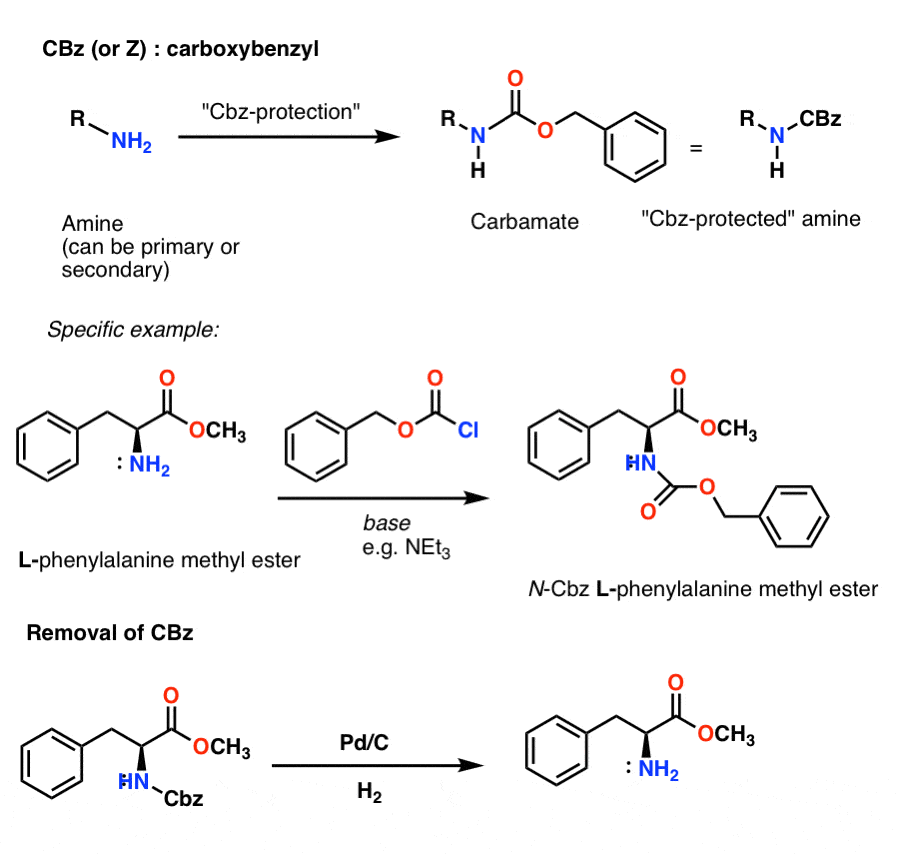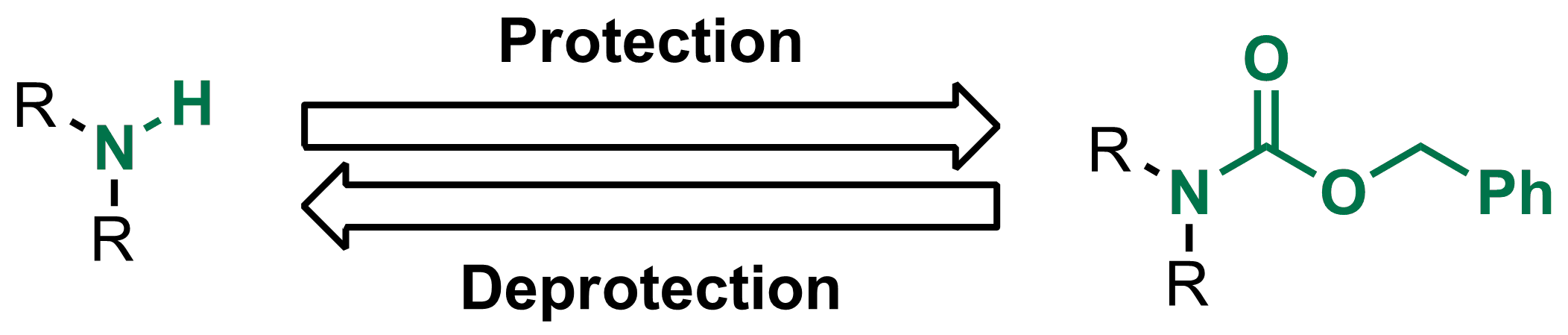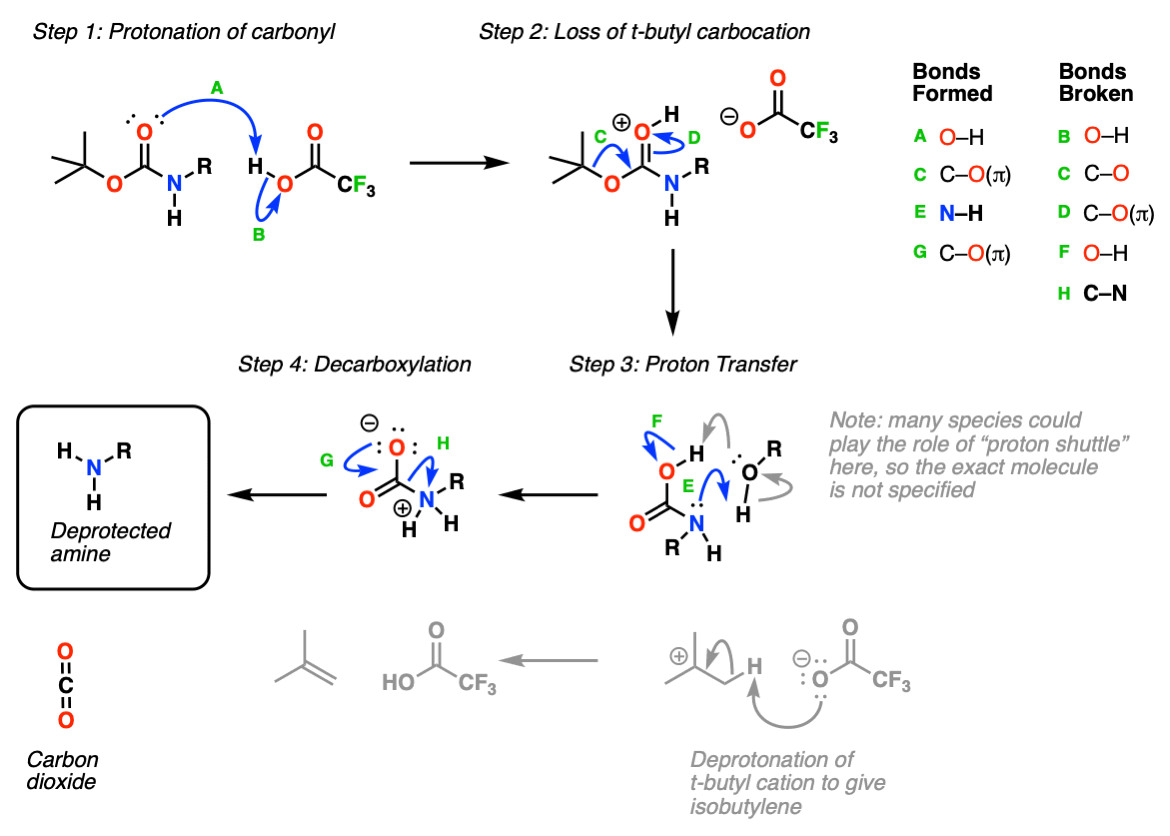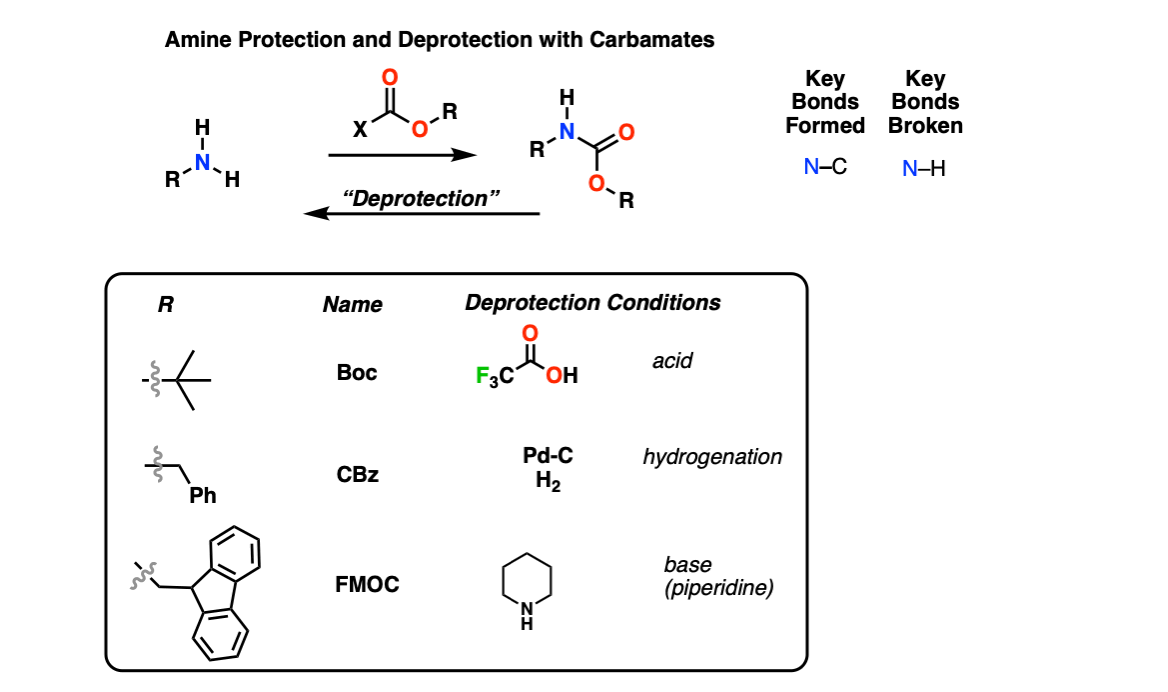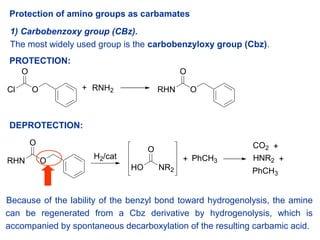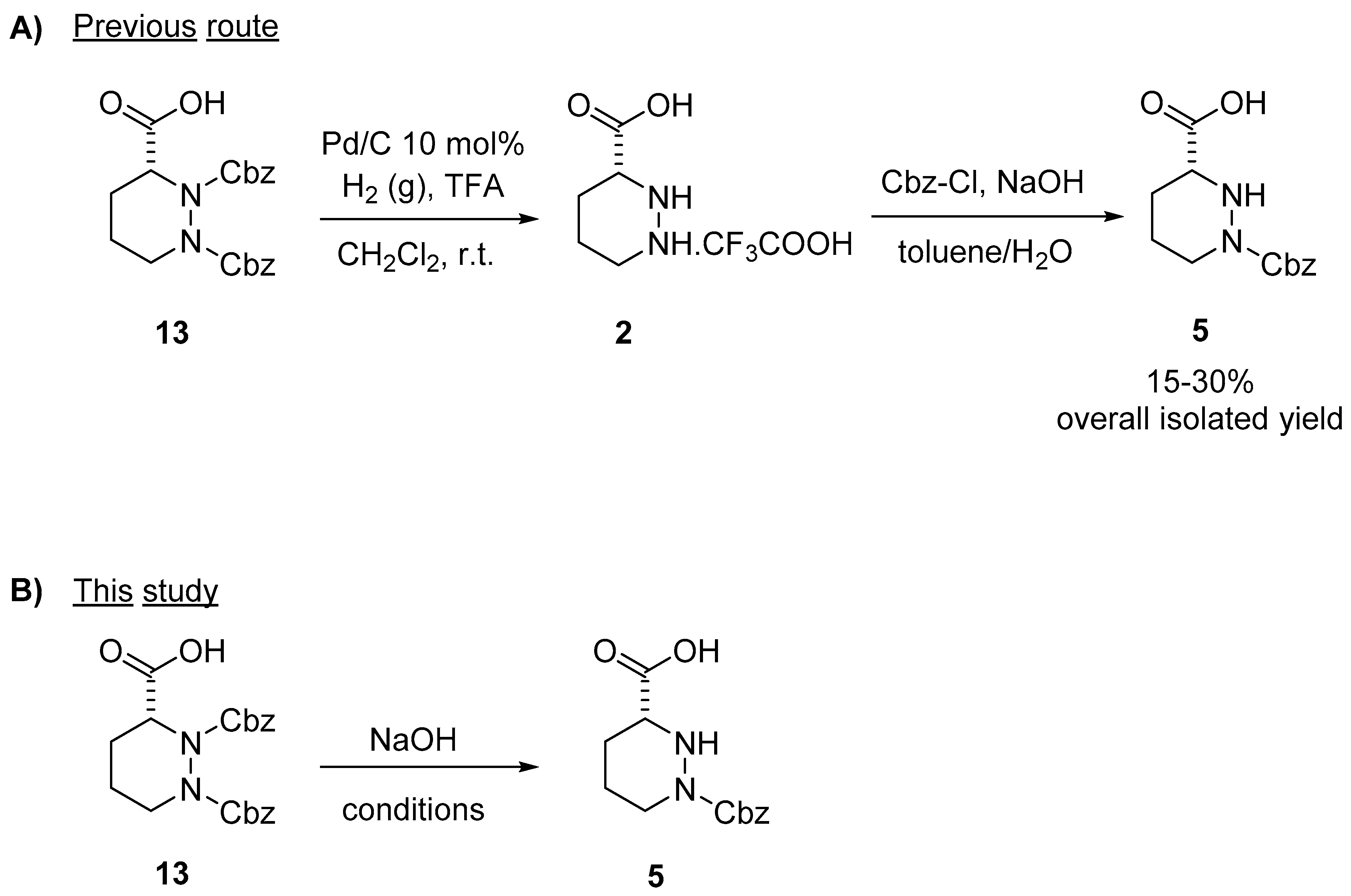
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML

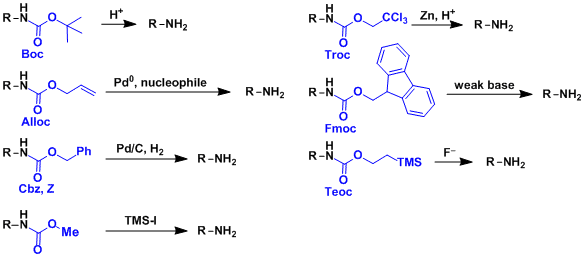
A convenient protocol for the deprotection of N-benzyloxycarbonyl (Cbz) and benzyl ester groups - ScienceDirect
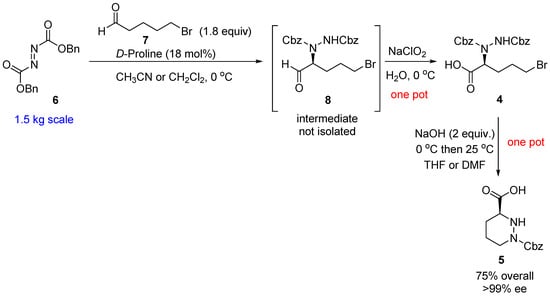
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML
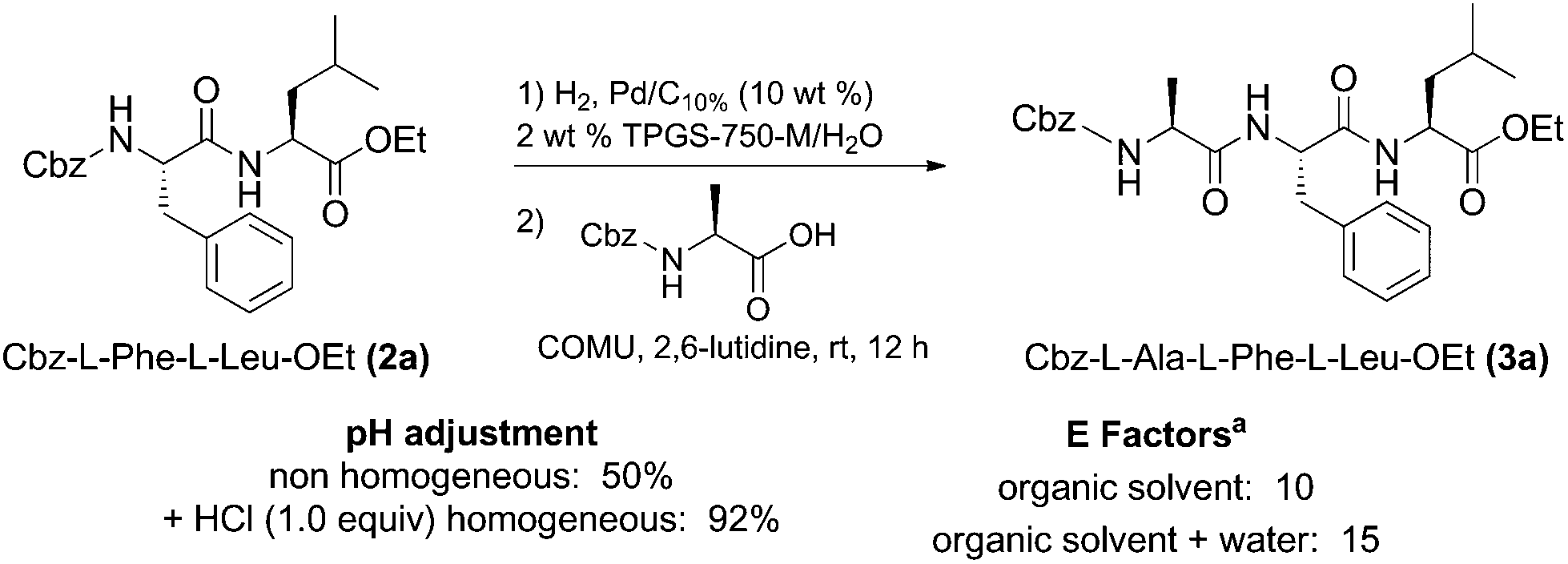
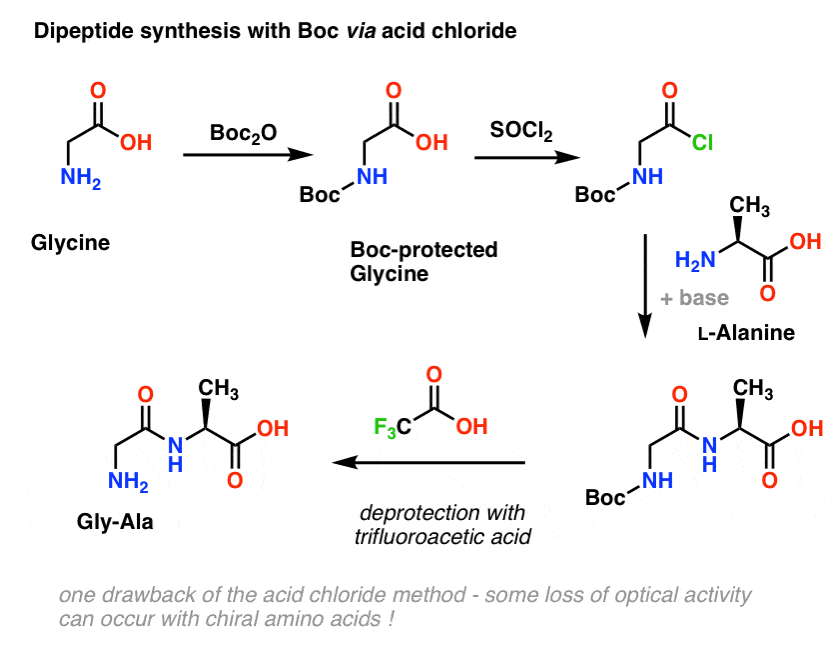
Tandem deprotection/coupling for peptide synthesis in water at room temperature - Green Chemistry (RSC Publishing) DOI:10.1039/C7GC01575E

Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML

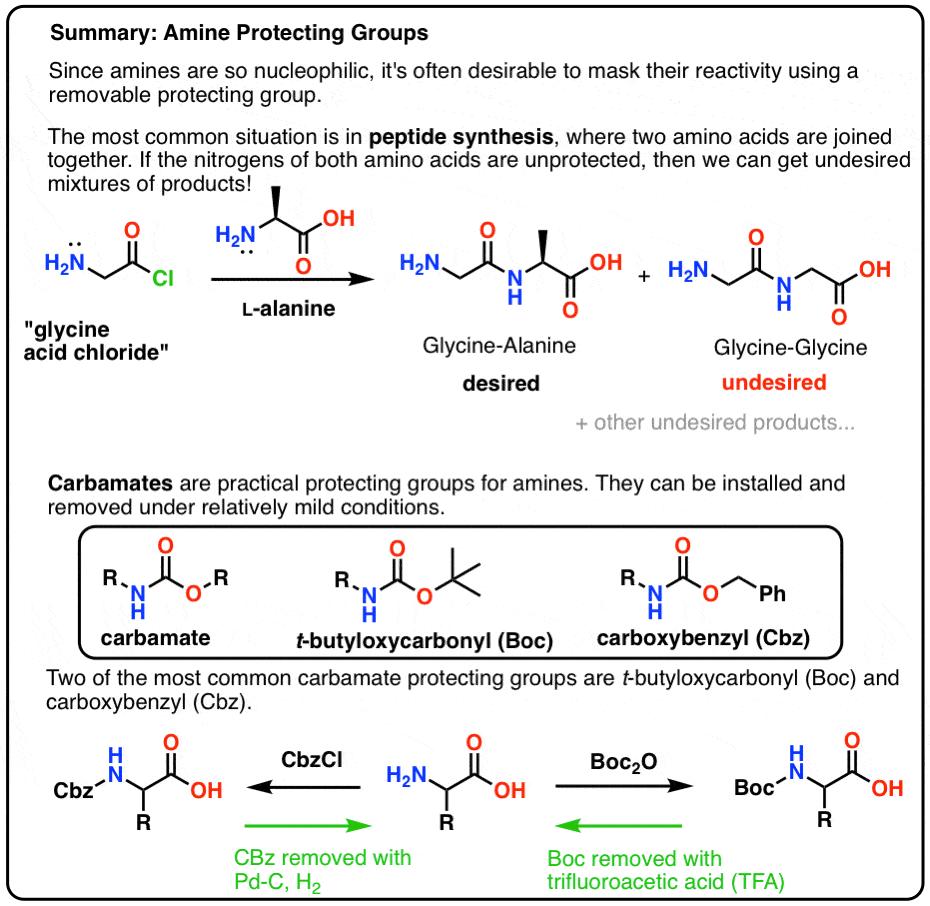
organic chemistry - Deprotection of carboxybenzyl (Cbz) in the presence of Methyl ester? - Chemistry Stack Exchange

A convenient protocol for the deprotection of N-benzyloxycarbonyl (Cbz) and benzyl ester groups - ScienceDirect
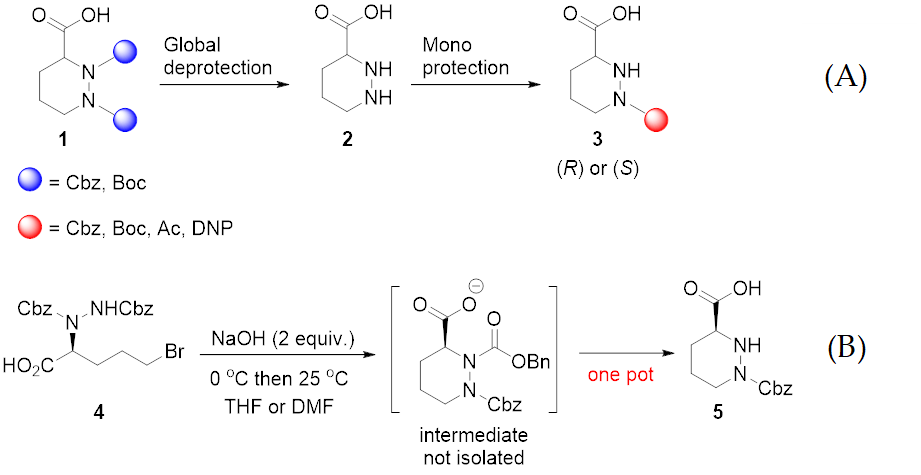
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML