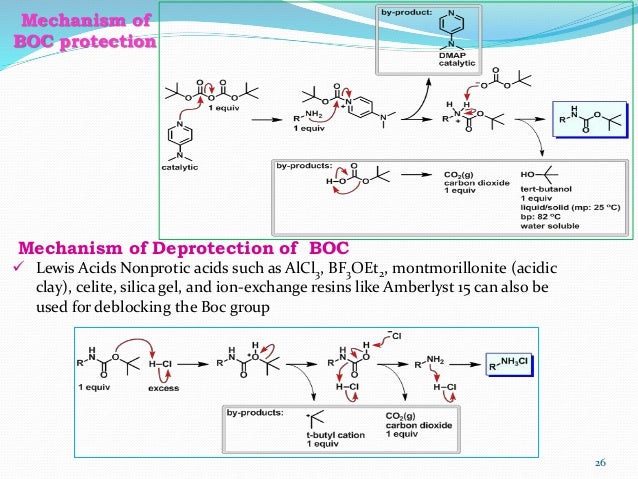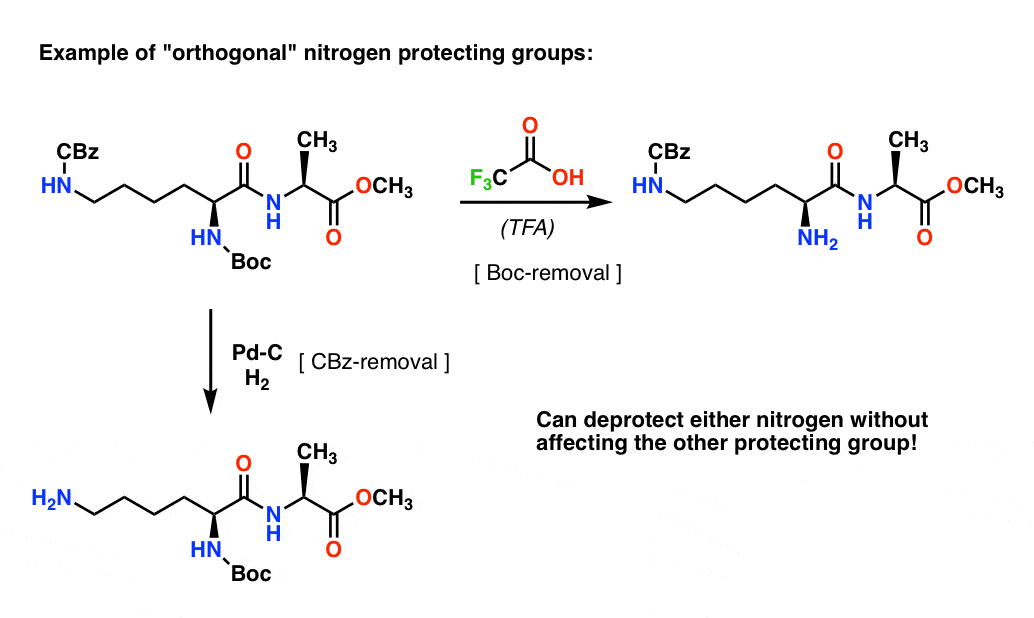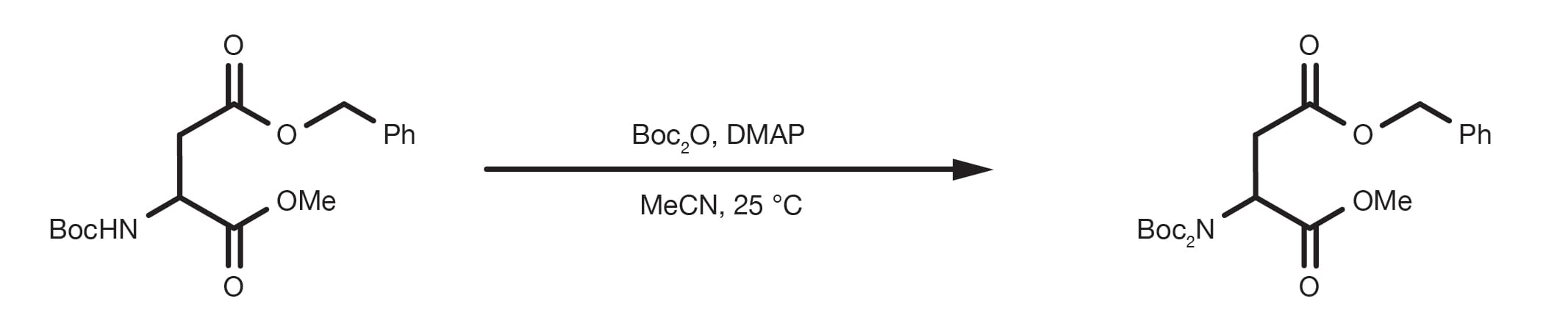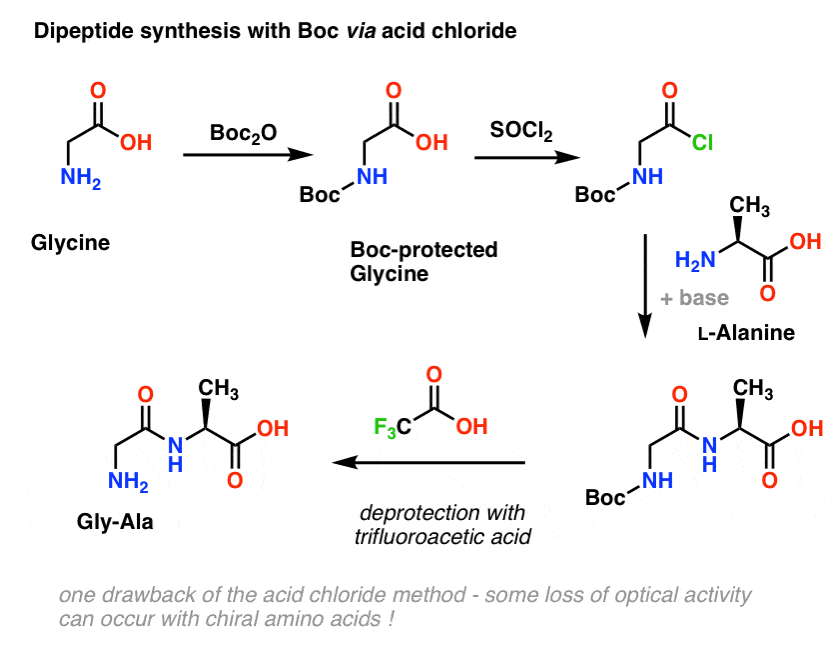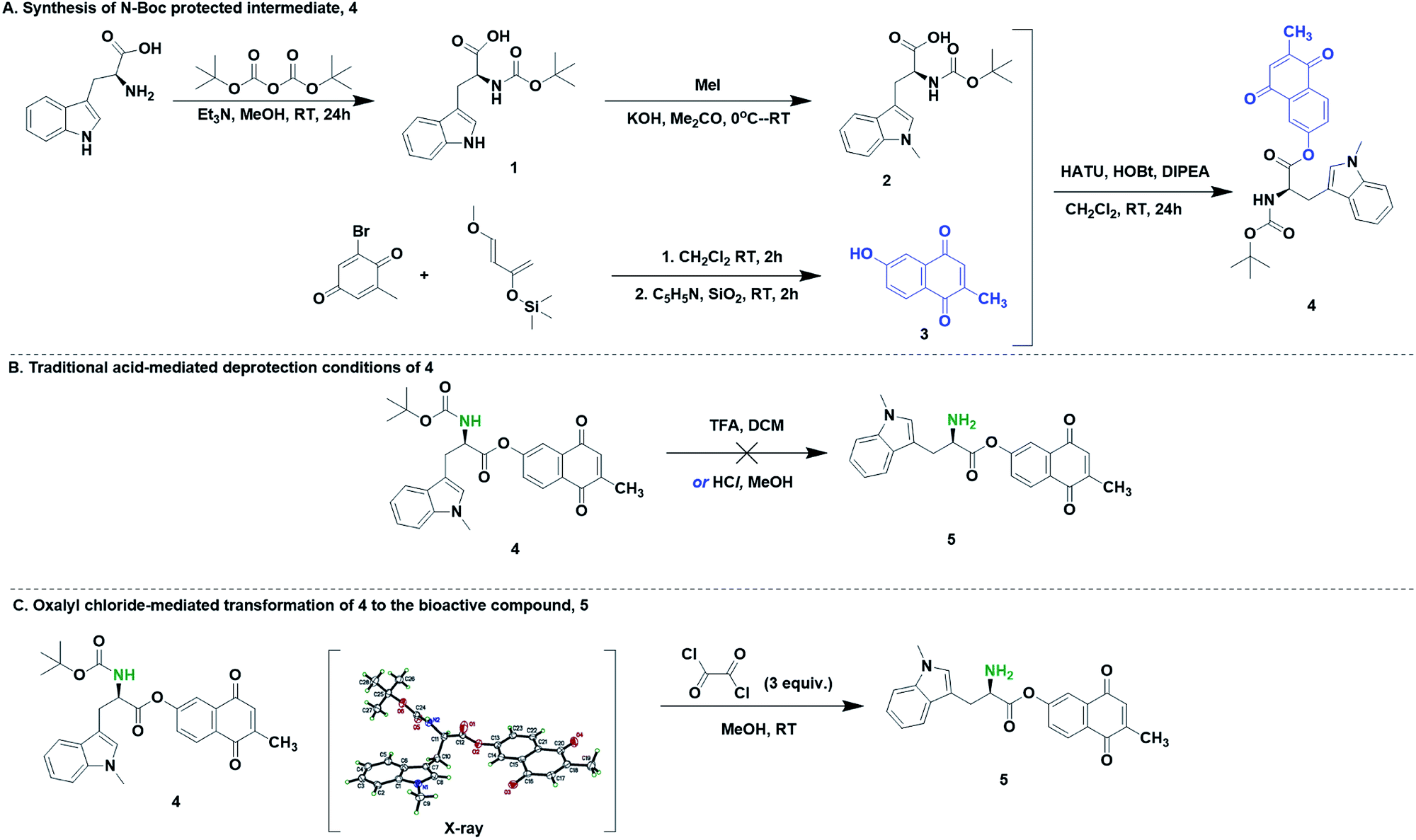
Mild deprotection of the N-tert -butyloxycarbonyl ( N -Boc) group using oxalyl chloride - RSC Advances (RSC Publishing) DOI:10.1039/D0RA04110F

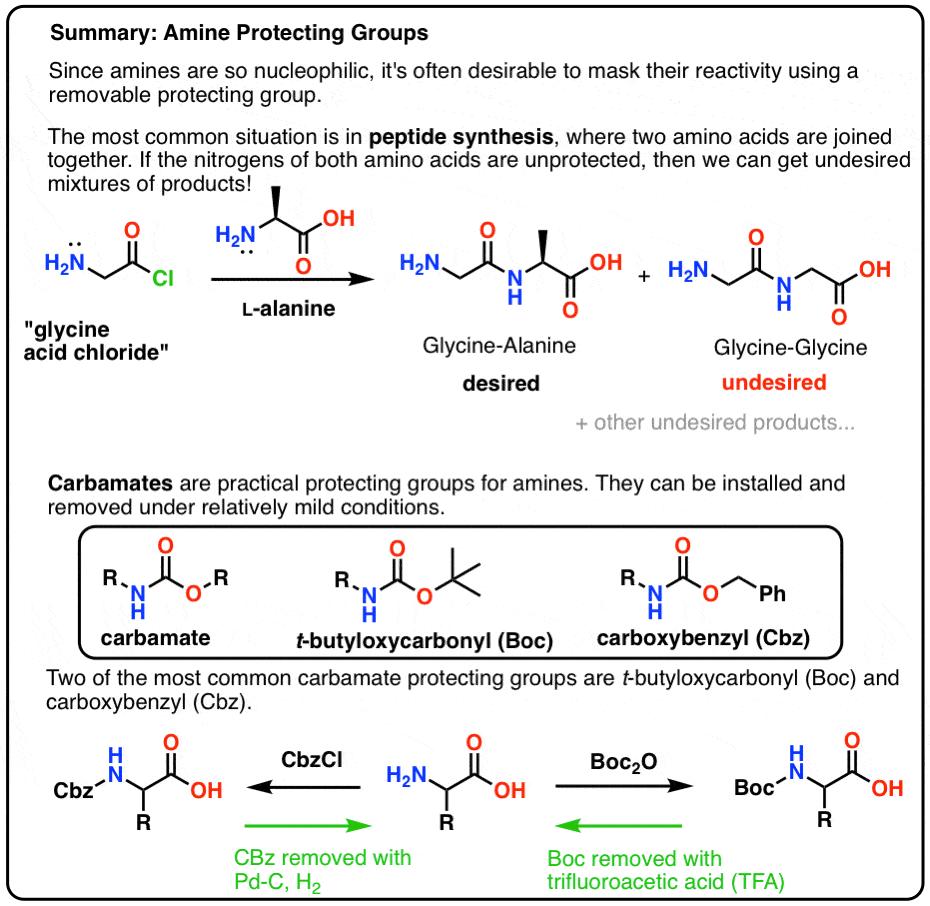
Tertiary-butoxycarbonyl (Boc) – A strategic group for N-protection/ deprotection in the synthesis of various natural/unnatural N-unprotected aminoacid cyanomethyl esters - ScienceDirect
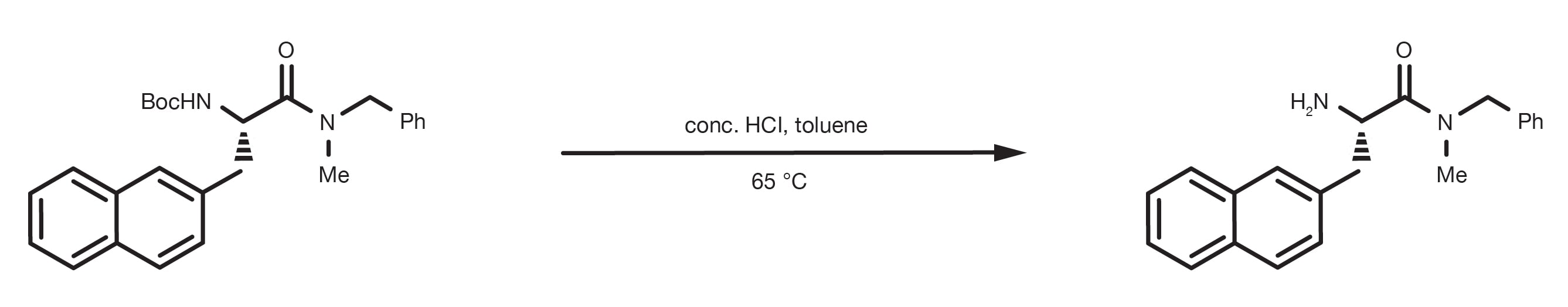
Rapid, effective deprotection of tert-butoxycarbonyl (Boc) amino acids and peptides at high temperatures using a thermally stabl