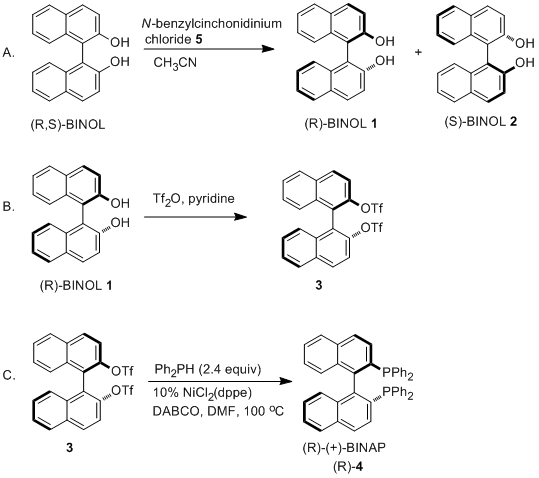
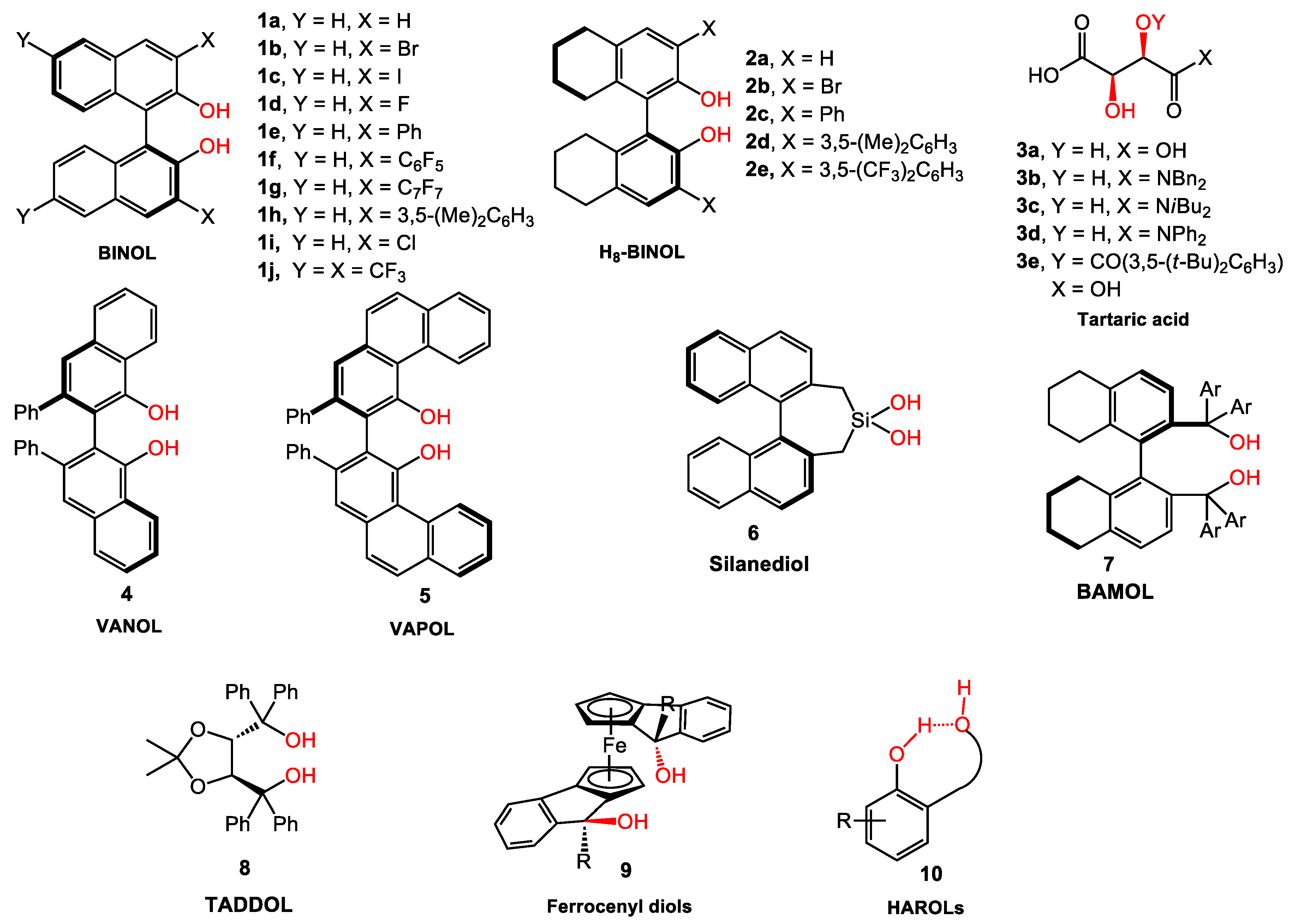
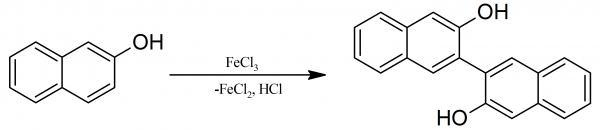
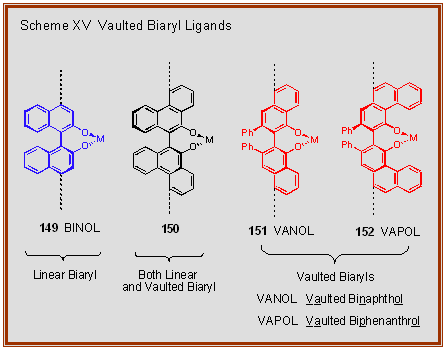
Feringa Lab on Twitter: "It may be 40 years old, but the boss's thesis is still useful when it comes to teaching BINOL synthesis to 1st year students..… https://t.co/92XkJf9eFJ"

Figure 2 | BINOL Macrocycle Derivatives: Synthesis of New Dinaphthyl Sulfide Aza Oxa Thia Crowns (Lariats)

a) Asymmetric reaction using (R)-BINOL derived chiral sulfur ylide;... | Download Scientific Diagram

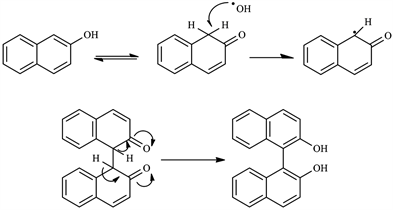
Recent advances in asymmetric oxidative coupling of 2‐naphthol and its derivatives - Wang - 2010 - Chirality - Wiley Online Library
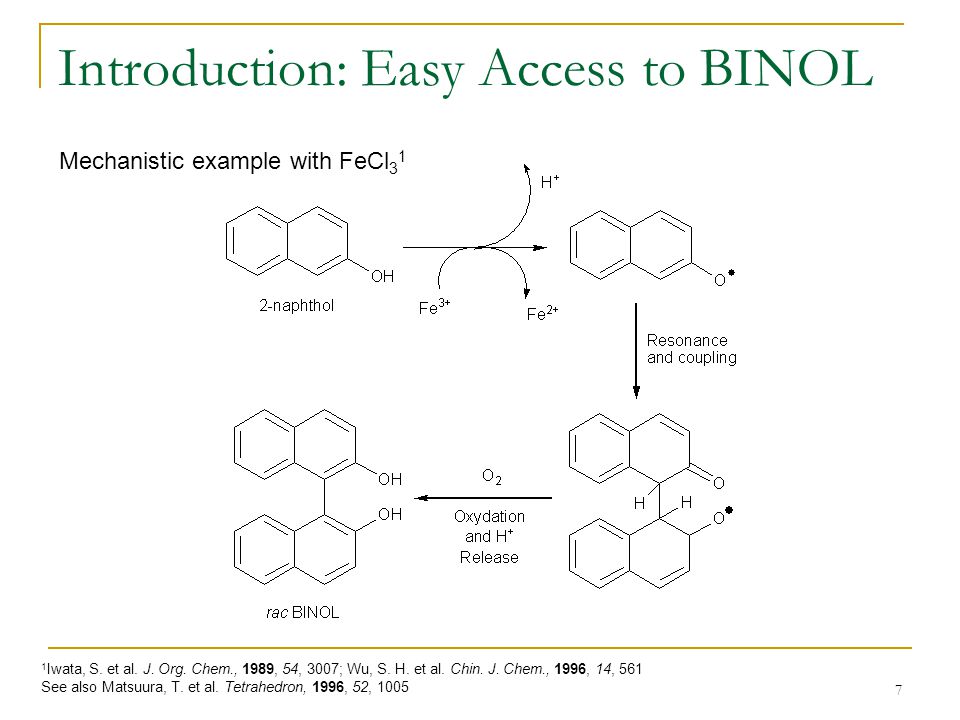
Applications of Atropisomerism: the Use and the Versatility of Enantioenriched BINOL Reagent in Organic Chemistry Literature Meeting, January 13th ppt video online download
Enantioselective Oxidative Homocoupling and Cross-Coupling of 2‑Naphthols Catalyzed by Chiral Iron Phosphate Complexes

Molecules | Free Full-Text | Enantioselective Iron/Bisquinolyldiamine Ligand-Catalyzed Oxidative Coupling Reaction of 2-Naphthols | HTML
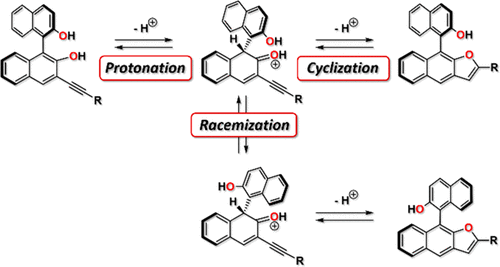
Synthesis of Furan-Annelated BINOL Derivatives: Acid-Catalyzed Cyclization Induces Partial Racemization,The Journal of Organic Chemistry - X-MOL

Synthesis of the bifunctional BINOL ligands and their applications in the asymmetric additions to carbonyl compounds - ScienceDirect
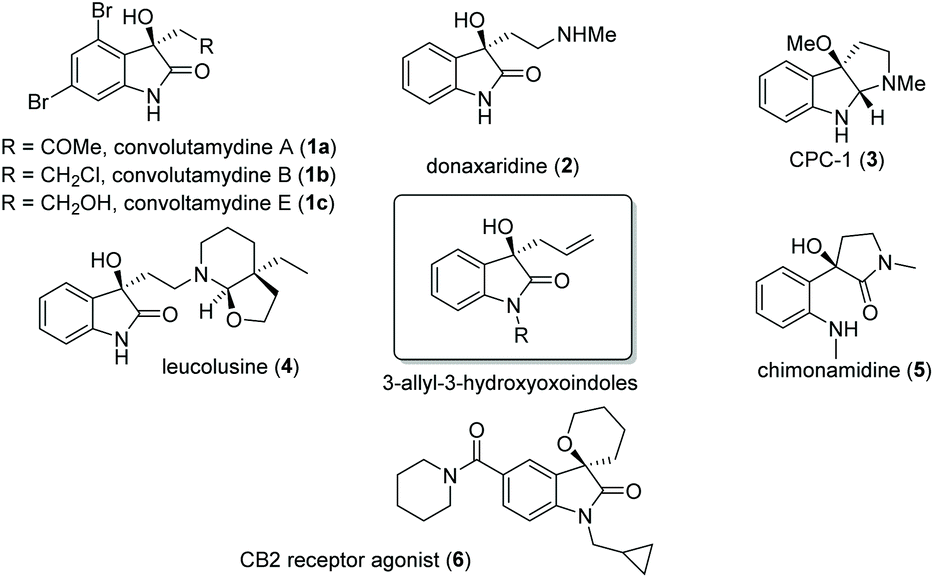
BINOL derivatives-catalysed enantioselective allylboration of isatins: application to the synthesis of ( R )-chimonamidine - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D0OB01386B

China High Quality BINOL Derivative: 1,1'-bi-2-naphthol CAS:602-09-5 Wholesale From China Suppliers and Manufacturers - Factory Wholesale - UCHEM
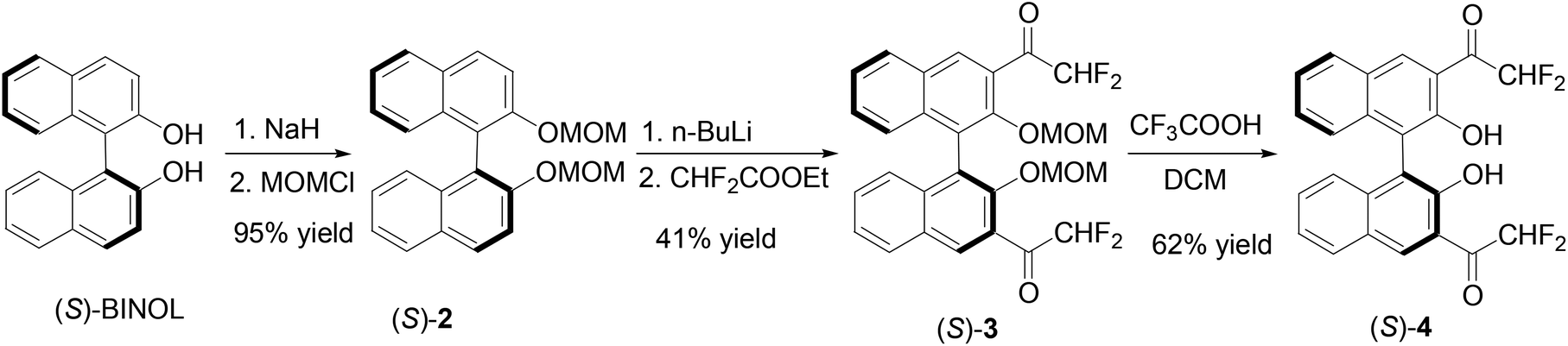
1,1′-Bi-2-naphthol-fluoroacetyl compounds in fluorescent recognition of amines - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C3QO00076A

Recent advances in asymmetric oxidative coupling of 2‐naphthol and its derivatives - Wang - 2010 - Chirality - Wiley Online Library
Preparation and Catalytic Properties of Various Oxides and Mesoporous Materials Containing Niobium and Sulfate Ions, in the Etherification Reaction of 2-Naphtol

Figure 3 | Copper(I)-BINOL Catalyzed Domino Synthesis of 1,4-Benzoxathiines through 𝐂 ( 𝐚 𝐫 𝐲 𝐥 ) -O Bond Formation